INTRODUCTION
Ruminants have adapted to feed on vegetables (forage) over thousands of years due to their evolution. This event led to the development of what is nowadays called rumen, a pre-gastric fermentation chamber composed of a range of microorganisms with the ability to degrade food, especially bulky or fibrous feeds, then releasing nutrients into the environment, which are used either for the host or ruminal microorganisms in a continuous process known as symbiosis 1.
Many animal breeds have emerged due to the strong human-made selection process over time, making ruminant animals more productive and also nutritionally more demanding. Thus, the supply of food for animals is no longer strictly based on forage, as animals also started to feed on grains. As the rumen environment is a fermentation chamber, this should be in anaerobic conditions, with pH close to neutrality (pH ±7) for its proper functioning. When changes occur to one or more of these basic rumen functioning aspects, there might be nutritional damages with direct impact to the production of milk, flesh and wool, as well as in the profitability of the livestock production system 2.
The rumen environment is composed of a range of microorganisms that can in general be divided into three large groups as follows: bacteria, protozoan and fungi. As the rumen environment is complex and always dynamic, depending on the environment conditions, may occur among microorganisms, seeking for resources for their own development. When diets with high proportion of grain are supplied to animals, the rumen equilibrium is compromised, since the pH of the environment may change from alkaline to acid, then affecting the development of ruminal microorganisms and, consequently, the use of food 3).
Bento et al 2 assessed the effect of the ruminal, abomasal and ruminal + abomasal infusions of casein associated with Tifton-85 hay based diet supplied to beef cattle on the ruminal and performance parameters. The values of pH, ruminal ammonia and synthesis of microbial protein in the rumen did not change (p>0.05). However, there was a significant effect (p<0.05) on the production of volatile fatty acids for animals supplemented with casein, when compared with animals under the control treatment.
To optimize the use of nutrients present in the diet of ruminants, it should better understand how different foods are degraded and fermented in the ruminal environment, in addition to the synchrony in the energy and proteins release, which have a direct effect on the final fermentation products and microbial population of the rumen environment. Therefore, this study aims at describing how different diets affect ruminal parameters and the microbial ecology of the rumen.
This study is based on qualitative and descriptive research, as per Koche 4 methodology. Information was collected from scientific journals indexed in national and international databases 5, with relevant information regarding the effect of the ruminant diet on ruminal parameters and rumen microbiota.
Ruminal microbiology and its modification as a function of different foods. Ruminants are animals with the ability to feed on forage and use nutrients present in vegetables for growth, reproduction and production of flesh, milk and wool. This ability for forage use is due to rumen, a fermentation chamber integrated by a diverse microbial population, essentially composed of bacteria, fungi, protozoa and archaea (methanogenic microorganisms), which occupy the liquid, solid, semi-solid and gas portions of the rumen 2.
Bacteria present in the rumen are divided into various genera, and they can be found in greater quantity and smaller volume of microbial mass, the cellulolytic and amylolytic bacteria, which can respectively degrade sources of fiber and carbohydrates (e.g. starch). The main structural carbohydrate fermenting bacteria are the following: Ruminococcus flavefaciens, Ruminococcus albus, Fibrobacter succinogene and Prevotella ruminicola. The most predominant group of amylolytie bacteria is Streptococcus bovis, followed by other amylolytie species such as Bacteroides amylophilus.
Fungi are usually found in smaller amount and greater mass when compared with bacteria, mainly because the ruminal environment is anaerobic, and there is few species of fungi that tolerate the anaerobiosis. One of the main characteristic of fungi is the ability to produce enzymes that degrade phenolic compounds such as lignin, which can be complexed with cellulose and hemicellulose molecules, and consequently, release such molecules to be used as substrate for their development. The ruminal microbiota of animals fed on diets containing high proportion of bulky feeds consists of high population of fungi when compared with the rumen microbiota of animals fed on high proportions of the concentrate 6.
Protozoa are found in greater amount and biomass when compared with fungi. However, they are often found in smaller amount and greater biomass when compared with bacteria, which can develop on different substrates and also use bacteria as source of substrate. Some species of protozoa have motility (chemotaxis), and they can be found on the rumen wall and food particles, since they have the motility ability that allow them seeking for nutrients released in the surrounding environment 7.
The archaea, also known as methanogenic bacteria based on their structure, are a group of single-celled organisms, taxonomically unknown as bacteria, as they have differences in membranes, are able to ferment different sources of substrate, and require sources of carbon released during the fermentation process of other microbial groups for their full development. Population of archaea may be high when animals are fed on diets containing high proportions of bulky, since this food contains high content of available carbon (C) that can be used by methanogenic bacteria for fermentation, and generate the methane gas (CH4) as the final product 1.
Belanche et al 1 evaluated performance and ruminal parameters of dairy cows fed with two levels of crude protein (10% above the requirements and 20% below the requirements for ruminants), and two types of carbohydrates (soy pod and beet peel). They found that animals managed to adapt to the over and under protein intake regimens, with 29% reduction in milk production from animals bred below the protein requirements and increase in 9% for animals bred above the protein requirements, associated with beet peel. The ruminal microbiota was changed as function of the type of the diet provided to animals, with reduction of pH and depletion of cellulolytic bacteria and fungi from animals fed on starch-rich diets; and increase of cellulolytic bacteria, fungi and methanogenic bacteria in the rumen microbiota of cows fed on fiber-rich diets.
Chen et al 3 studied different ratios bulky:concentrate (B:C of 97:3, 60:40, 40:60, 25:75, 15:85 and 8:92) on the performance and production of beef heifers. They found constant changes in the ruminal microbial population, as well as in the microbial diversity during the adaptation process. The polymerase chain reaction (PCR) showed significant changes in the diversity of rumen microorganisms at 97:3 proportion, with a prevalence of microorganisms that degrade non-fibrous carbohydrates; while the microbial diversity increased at 8:92 proportion, also with higher production of acetic. These findings show that the greater production of acetic acid, high values of pH and the increase of microbial diversity were obtained from greater proportion of bulky feeds in the diet. Studies support this statement by showing that the diversity of microorganisms that degrade sources of fiber is significantly greater than those from microorganisms that degrade sources of non-fibrous carbohydrates 8.
Table 1 shows the effect of ratios bulky:eoneentrate (B:C) on the behavior of the rumen microbial population and on the production of volatile fatty acids. It observes that the increase in the proportion of fiber in the diet provided to animals results in a significant increase of the amount of bacteria, fungi and protozoa in the rumen 9, once fibrolytic bacteria usually act in greater number of species when compared with amylolytie bacteria, under favorable ruminal fermentation conditions. Thus, bulky-rich diets show greater microbial diversity, as well as greater occurrence of fungi and protozoa in the rumen 1.
Table 1 Effect of different proportions of bulky:concentrate (B:C) on the microbial population of the rumen and percentage of total bacteria.
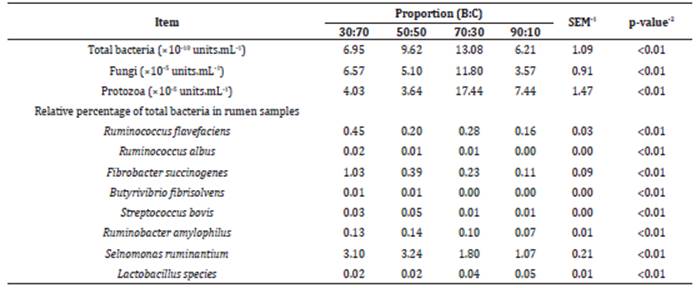
1-SEM: standard error of mean; 2-p-value: a measure of the probability that an observed difference could have occurred just by random chance, at significance level of 0.05. Source: Adapted from Ding et al 9.
The entire microbial diversity found in the rumen is dynamically modified, since microbial groups present in the rumen are in constant competition for substrates. The development of a particular microbial group, which usually occurs at the expense of another group of microorganisms, depends on the conditions of the environment and the available substrates. The multiplication of these microorganisms occurs as function of degradation of food particles found in the environment, which are further fractionated into small sizes until they become monomers that can be used in the fermentative pathway of ruminal microorganisms 8.
Depending on the metabolic pathway used, bacteria can produce distinct final fermentation products in the rumen, such as the releasing of volatile fatty acids, mainly the acetic, butyric, propionic, isobutyric and isovaleric acids; as well as free molecules of carbon, ammonia and hydrogen ions. Animals can use acids produced in the rumen by means of the absorption ability via ruminal epithelium and further use them via hepatic, thus participating in metabolic cycles that generate energy as the adenosine triphosphate (ATP) for the animal 10.
The rumen environment is considered a dynamic system, with the pH close to neutrality and the anaerobiosis as the main characteristics for its perfect functioning. The rumen as a fermentation chamber shows, in ideal conditions, a pH from 6.8 to 7.2, which is found to be an optimal pH for the development of a wide range of microorganisms. The ruminal pH may change as function of the diet and characteristics of the foods provided to the animal. When the pH is above the maximum recommended level, the ruminal alkalosis may occur, and a pH below the minimum recommended limit may cause the ruminal acidosis. The anaerobiosis condition in the rumen environment is also found to be essential, since most microorganisms found in the rumen are obligate anaerobes, which means microorganisms development in the rumen may cease in the presence of oxygen 9.
The rumen fermentation from ruminants that use forage plants as the basis for their feeding is predominantly associated with microorganisms that degrade sources of structural carbohydrates and low release proportion of hydrogen ions (H+), since the amount of acetic acid from the fermentation process is greater when compared with the amount of butyric and propionic acids. The acetic acid has lower acidification capacity when compared with the propionic acid. The conversion process that occurs during the breaking of glucose bonds, a six carbon ring, which will further be fermented by bacteria and produce two molecules of acetic acid, uses four atoms of carbon. This process allows releasing two atoms of carbon for the environment, which may be used by other microbial groups such as archaea, to generate CH4 as the final fermentation product 11.
When animals are fed on diets containing high proportions of grains, sources of non-fibrous carbohydrates, amylolytic bacteria from the rumen develop in greater proportion, resulting in an intense production of propionic acid as the final fermentation product. During this conversion process, a molecule of glucose is converted into two molecules of propionic acid, a fatty acid consisting of three carbon molecules; with no loss of energy. Thus, this acidification of the ruminal environment, which is associated with the limited buffering capacity of ruminants feed on diets with high proportion of grains, may trigger metabolic problems such as ruminal acidosis 12.
The microbial population in the rumen changes either as function of different proportions of B:C or as function of other substances known as sources of fatty acids that can cause changes to the ruminal microbiota. The supply of sources of oil in the rumen may affect the development of bacteria. Huws et al 13 assessed the inclusion of fish oil in the diet of heifers and found that the microbial diversity in the rumen, the synthesis of microbial protein, as well as the digestibility of experimental diets, reduced when the content of oil in the diet was increased.
Kim 12 assessed the effect of the feeding regimen based on two different diets as follows: the diet #1 consisting of Timothy Grass hay for Jersey cows, and the diet #2 consisting of Timothy Grass hay + grains for Dutch cows. Authors measured the effect of treatments on ruminal parameters and rumen microbiota, comparing unculturable bacteria in culture media with culturable bacteria. By using the polymerase chain reaction (PCR), they found that animals fed on the diet #1 showed greater amount of total microorganisms on the rumen wall, from which most were unculturable bacteria. On other hand, animals fed on the diet #2 showed low amount of total microorganisms, from which the majority were culturable bacteria and associated with the liquid part of the rumen fluid.
Lillis et al 14 used the real-time PCR to assess the effect of the proportions B:C = 50:50 and B:C = 90:10 on the microbial diversity, amount of methanogenic bacteria and ruminal parameters of beef steers. They found that animals fed on diets consisting of the proportion B:C = 90:10 showed lower microbial diversity, greater synthesis of propionic acid than acetic acid, and lower population of methanogenic bacteria. On other hand, animals fed on diets consisting of the proportion B:C=50:50 showed greater microbial diversity, greater synthesis of acetic acid than propionic acid, and greater population of methanogenie bacteria.
Findings described above show how the rumen microbiota varies according to changes made to the diet provided to animals. Studies described here show that the increasing of the proportion of bulky feeds results in the increasing of the microorganism population, greater microbial diversity and lesser production of propionic acid than acetic acid, and greater release of methane to the atmosphere. However, animals fed on grains-based diets show greater synthesis of propionic acid, pH tending to acid, lower microbial diversity, and lower use of fibrous sources present in the diet 14.
Microbial competition in the rumen environment. Taking into consideration the particularities of the rumen, it knows that different genera of bacteria prevail in the rumen environment, and each bacterial species shows a certain tendency for increasing or decreasing its multiplication rate, according to the conditions of the rumen, such as temperature, pH, absence of oxygen, and the available source of substrate 12.
Feeds containing high proportion of non-fibrous carbohydrates, such as starch, are rapidly fermented in the rumen. In addition, due to the weak cohesion of their bonds, bacteria require a shorter colonization time to degrade particles of food, thus succeeding to multiply rapidly and modify conditions of the environment, releasing more acid (greater acidifying power), and inhibiting the multiplication process of other opportunistic microbial groups 3.
Diets consisting of high proportions of bulky allow vast colonization of the rumen by cellulolytic and hemicellulolytic bacteria, fungi and also protozoa. The time a bulky food will remain in the rumen to be better used is greater when compared with the time a concentrate food would remain. However, the amount of non-degradable protein in the rumen, which will be available at the intestinal level, will also be greater. As this multiplication is slow, exclusive sources of bulky feed rarely meet all the protein and energy requirements that animals need on a daily basis. Thus, supplementing animals with sources of non-fibrous carbohydrates such as maize, wheat, barley, soy, among other grains and cereals used for animals feeding is founds necessary 15.
Changes in the ruminal pH may propitiate the development of pathogenic microorganisms such as Escherichia coli, which has high growth rates, multiplies rapidly, and suppress the development of other microbial groups. This microorganism has high degree of pathogenicity, as it may sporulate and produce substances that cause animal metabolic disorders, diarrhea, lack of appetite, hemorrhages and other problems; which can even contaminate the milk and meat of exposed animals 16.
Chen et al 17 assessed ruminal parameters and the microbial population in beef steers fed on two diets, one based on high-grain and another on high-bulky. They found that animals fed on diet consisting of high-grain showed subclinical acidosis, ruminal pH below 5.0, as well as reduction in microbial groups fermenting of structural carbohydrates, decrease of microbial diversity. On other hand, animals fed on the diet consisting of high proportion of the bulky, showed a ruminal pH close to neutrality, from 5.89 to 6.40, and high development of cellulolytic and hemicellulolytic microorganisms.
Studying how microbial groups behave in the rumen, as function of different foods and proportions of B:C is essential for better understanding of these processes, in order to achieve better results from the use of food by animals. With current technological advances, studies using genetic analysis by PCR have been conducted aiming at assessing relationships and development of ruminal microbiota, which usually differ from other studies on rumen microbiota based on cultural methods. For PCR analysis, 16S rRNA and 18S rDNA subunits of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) obtained from microorganisms present in the medium were extracted for further amplification using selected primer-pairs. The cycling parameters for PCR amplification consisted of initial denaturation step, followed by denaturation, annealing, initial extension and a final extension. PCR products were then compared with nitrogen bases from the database, which are sufficiently similar to nitrogen bases of interest that likely share a common characteristic. Findings show that the molecular biology may lead to advances on ruminal ecosystem studies, although this technique could not identify all microorganisms that inhabit the rumen environment, since most of them are quite sensitive to conditions outside the rumen 18.
Ruminal parameters. The intensification of animal production systems led to an increased risk for metabolic disturbances either for dairy or beef herds, resulting from the inclusion of high content of grains in the diet. The key factor for this occurrence is the desire for intensification of the production system, searching for better production rates, which result in imbalance between nutrients supply and production levels demanded by producers 19.
Studies show that the best environmental conditions for the development of ruminal microorganisms are temperature at 39oC and ruminal pH around 6.7 20,21,22. However, when these conditions are not met due to management errors, for instance, the intake rate may reduce, and then the weight gain and milk production. In addition, changes in the milk composition may occur, and mainly metabolism disorders, which have a direct effect in the production costs of the unity and also causing losses to rural producers 22.
The increase in the production leads gradually to the increase of eases of metabolic disorders, which are in some eases described as metabolic diseases. Such disorders are characterized by biochemistry changes occurring in the urine, ruminal fluid and blood, followed by the production reduction around 10 to 25% and the emergence of reproductive problems, although animals may look apparently perfectly healthy. Thus, by knowing the processes that occur at the ruminal level and how the rumen in different situations, as well as the composition of feeds, is essential for the identification of the limit for adjustments in the feeding, without causing any damage to the animal 23.
The rumen-reticulum is the main compartment where the dietary digestion in ruminants occurs, as well as actions performed by the most diverse and numerous microbial populations take place 1,24. During the intake of food by the animal, the rumen receives the essential nutrients for microorganisms' growth, which provide the animal with volatile fatty acids (VFAs) originated from the fermentation of feed and releasing of substrate that will be used by ruminal microorganisms. These microorganisms multiply in food, producing the microbial protein that will supply nutrients to be used in the animal intestine, and will be source of substrate for other microorganisms in the rumen. Microorganisms produce VFAs as the final fermentation products, which are usually absorbed in the rumen wall and used as energetic source for ruminants 25.
Ruminal microorganisms are responsible for fermentation process and they require conditions that favor the ruminal dynamic. The following parameters are commonly used to assess the ruminal functioning: pH, ruminal fluid, content of nitrogen in the rumen, molar concentration, proportion of VFAs produced in the rumen, and the amount of microorganisms 19.
The assessment of the ruminal dynamic present in the diet promotes fermentation changes, characterizing requirements of herds with possible changes made to the nutritional management system. The fermentation pattern is an indication of the potential nutritional value that food would promote better performance. On other hand, the pH, content of ammonia and VFAs produced and absorbed in the rumen are indicators of ruminal environment conditions, while estimates of the intake and digestibility describe the efficiency in the utilization of the food 26.
Ruiz-Albarrán et al 21 assessed the effect of type of the silage on the ruminal metabolism of dairy cows, and they found that the efficiency of the ruminal microbial protein improved as the supply of forage to animals increased. However, the silage provided lesser effect on the production of milk and rumen fermentation.
Converging results were obtained by Zhu et al 22, who tested the effects of the products of fermentation with Saccharomyces cerevisiae on the performance, the rumen fermentation and the ruminal microbiota of dairy cows fed on a diet consisting of low quality of forage. These authors found that the supplementation with S. cerevisiae modified the microbial population of the rumen for a greater nitrogen energetic efficiency of animals.
Ruminants with high productive potential are usually fed on energetic diets based on cereal, from which, the rapid ruminal fermentation of starch and sugar may cause ruminal acidosis, a metabolic disorder that exists in both acute and subacute forms 1,19. This acidosis occurs due to the decrease in pH as a result of accumulation of organic acids and modification in the balance of rumen microorganisms, in which the growth of not acid-tolerant microorganisms stops and, consequently, the microbial competition reduces, and environmental conditions become conducive only for acid-tolerant microorganisms 24.
Benehaar et al 24 assessed the effect of the supplementation of dairy cows with increasing amount of linseed oil added to the ration on the digestion, characteristics of ruminal fermentation and population of protozoa. They found that the addition of 4% linseed oil may be safely made to forage-based diet for lactating cows for milk enrichment to the benefit of the animal health, without causing any harmful effect on the digestion, rumen function.
Therefore, the assessment of ruminal parameters in experiments with animals is noteworthy, since understanding how each parameter behaves in function of different feeding situations, may contribute to minimize or prevent metabolic disorders to the animals.
Factors that affect the rumen pH. The rumen consists of a diversified microbial ecosystem, which transforms fibrous plant material and non-protein nitrogen into products such as short-chain fatty acids and microbial protein 22. The rumen epithelium absorbs VFAs, what is found to be essential to prevent the accumulation of H+ ions and the consequent reduction in rumen pH. When animals are fed on diets containing high amount of non-fibrous carbohydrates without prior adaptation, some physiological processes are activated, resulting in metabolic disorders such as ruminal acidosis 26.
The ruminal pH is usually related to final fermentation products. Then, after the intake of food with rapid fermentation rate and the digestion, the reduction of pH and consequent acidification of rumen may occur 24. This fermentation process provides sources of organic acids such as acetic and propionic acids, which can be used by ruminants as sources of energy, followed by the synthesis of non-beneficial products such as methane 24.
Thus, an adequate rumen buffering must occur to maintain levels of ruminal pH close to neutrality. In case of conventional diets with no addition of ruminal manipulators, the important buffering agent used is the animal saliva, which has satisfactory concentrations of bicarbonate and other minerals, preventing the rumen pH to reach values below 5.0 27.
The pH of the ruminal fluid must range from 6.0 to 7.0 to allow rumen proteolytic activities to occur. However, the ideal pH is 6.5 for most microorganisms, and its maintenance is important for maximum cellulolytic activities 21.
When the production rate of VFAs exceeds the absorption capacity of the ruminal epithelium, the accumulation of acids in the rumen may occur, resulting in pH decreasing. This process promotes changes in the fermentation patterns of the rumen microbial population, where the lactic acid, which has stronger acidity than other acids due to the high dissociation constant, acts by further reducing the ruminal pH 19.
The assessment of the ruminal pH is important since it affects directly the characteristics of the animal diet. Then, changes in this parameter may affect the growth rate of bacteria and protozoa 28. The stabilization of the pH is, in general, attributed to the saliva, due to its buffering function. In addition, the ruminal mucosa has the ability to absorb acids produced during the ruminal fermentation.
When the ruminal pH is below 6.0, changes in the production of microbial protein and VFAs may occur, leading to intake decrease and lower synthesis of milk, as well as changes in its composition, which can lead to metabolic disturbances and lower productive profitability 19,29.
Sun et al 23 studied the effect of Bacillussubtilis natto on milk production, ruminal fermentation and ruminal mierobiota of dairy cows, and found that the supplementation with B. subtilis natto increased the population of ruminal bacteria, especially amylolytic, proteolytic and total bacteria, reduced the amount of protozoa and ruminal fermentation, and increased the total concentration of VFAs.
The microbial growth in the rumen is intrinsically related to the animal digestive capacity, and influences the production of VFAs and the flow of microbial protein to the small intestine. The microbial efficiency is usually assessed by the production of microbial cells synthesized per unit of the substrate used. Factors that affect the microbial efficiency are the availability and the synchronization between the energy and nitrogen compounds. The availability of ammoniacal nitrogen (NH3-N) may be the factor that restricts the microbial growth in the rumen (30). Thus, the ratio bulky:concentrate in the diet provided to animals has a direct effect on the pH, ammonia and VFAs in the rumen (Table 2).
Table 2 Ratios bulky:concentrate (B:C) and its effects on pH, ammonia and volatile fatty acids in bovines.
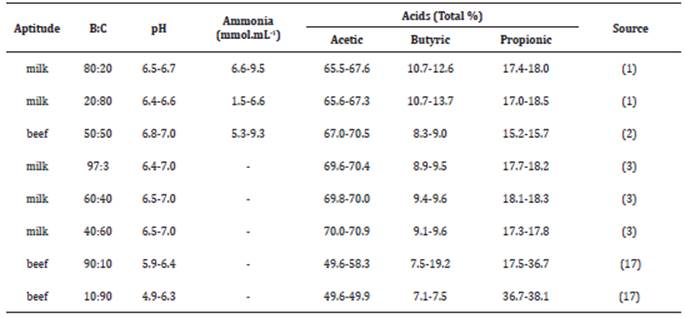
The supply of nitrogen in the rumen, in addition to other essential nutrients, may limit the microbial fermentation. The ruminal ammonia is an indicator of N-use efficiency by the animal, since around 60% to 80% of N absorbed by microorganisms is from ammonia 15. The ruminal concentration of ammoniacal nitrogen (NH3-N) results from the balance between its production and utilization by ruminal microorganisms 28.
Rumen microorganisms act in the degradation of protein sources, by producing NH3-N. The ruminal ammonia originates from non-protein N in the diet, degradation of true protein and also from the recycling by saliva or diffusion through the rumen wall. On other hand, its removal occurs by incorporation of microbial protein or ruminal absorption 22.
The NH3-N present in the rumen is assimilated by microorganisms through two main pathways, a follows: from the glutamate dehydrogenase enzyme, and by glutamine synthetase and glutamate synthase enzymes. The first pathway acts under high concentration of NH3-N without wasting energy, and the second prevails under low conditions of NH3-N; however, with energy expenditure 27.
The subacute ruminal acidosis occurs in diets for ruminants either with low or high energy, and the digestible energy is provided by highly fermentable carbohydrates, mainly at pH below 5.6 21. A decrease in ruminal pH may also cause changes in the production of VFAs, causing consumption decrease and resulting in low production of milk and low weight gain. The ruminal pH is negatively related to the concentration of VFAs in the rumen, i.e., when the pH is high, the absorption reduces and vice-versa 30.
Ratio bulky:concentrate and its influence on microbial concentration and final fermentation products. The feeding supplementation influences directly the concentration of ammonia and the pH of the rumen fluid, favoring the microbial population, which alters degradation mechanisms of the fibrous fraction of forage, as well as the synthesis of nutrients in the rumen 14. Formulation of diets containing different levels of the concentrate modifies the rumen biochemistry and show a direct effect regarding the consumption, contributing to satisfactory results in a feeding program 24.
The maximization of the efficiency of the microbial synthesis has been altered by manipulating the ratio bulky: concentrate, which can affect the production regarding the microbial efficiency of the rumen, enhanced by the availability of the substrate and ruminal pH 27.
There is no consensus regarding the best ratio bulky:eoneentrate, since some studies report that the higher microbial efficiency was found at the ratio B:C = 80:20, while better results on the microbial production were found on diets formulated at the ratio B:C = 38:62 19.
Animals raised in tropical conditions occupy pasture systems during the most part of the year, systems in which variation in growth and nutritional value of forages may occur, requiring an additional supply of nutrients to meet the energetic requirement for animals 29. During seasons with forage scarcity, the management of the supply and supplementation with concentrate or silage are common practices that determine the animal nutritional state 20.
The rumen pH is, among ruminal fermentation parameters, related with various processes that occur in the rumen. The pH decrease affects negatively the dry matter intake, ruminal motility, degradation of the fibrous fraction and microbial production; compromising the perfect functioning of the rumen and, consequently, affecting directly the animal health 25.
When lactating animals are fed on diets containing high proportion of grass silage, the concentration of protein in the milk tends to reduce, probably due to the reduced energy intake or low retention of N from the silage, when compared with diets consisting of concentrate feeds 21.
The effect of the application of fibrolytic enzymes in diets containing low and high levels of concentrate on the performance of lactating dairy cows has studied 17. These authors found that the supply of fibrolytic enzymes did not affect the daily intake of dry matter and production of milk. However, the availability of nutrients and the total concentration of VFAs increased. Regarding milk production and digestibility of dry matter, they also found that the application of these enzymes on diets of dairy cows with low concentrate content caused similar effects from those found for animals fed on diets containing high concentrate. These benefits were likely due to the effect of the enzyme in the improvement of nutrients digestion.
There is a high variation on molar proportions of acetate:propionate:butyrate in the diet. Thus, when animals are fed on diets rich in fibrous carbohydrates, the proportion 75:15:10 may be found, while the molar proportion 40:40:20 is found when animals are fed on diets rich in non-fibrous carbohydrates; with the total VFAs varying from 60 to 150 nM.mL-1 of the ruminal fluid 31.
According to Foiklang et al 28, the importance of increasing the efficiency of nutrients use by ruminants encourages studies to assess balanced diets, taking into account the existing relationships between ruminal microorganisms and their hosts.
Therefore, there is a negative relationship between the ruminal concentration of VFAs and the rumen pH, due to the high variation of diets effect in the removal and neutralization of rumen acids, which likely affects such relationship. Dijkstra et al 32 studied the regulation of rumen pH and consequences of a low pH in the rumen. They found that the reduction of ruminal pH is associated with the decrease in fibers degradation, as well as in the ratio acetate:propionate and methane production. These authors also stated that increasing the supply of dietary proteins in the diet is found to be necessary, to allow the obtaining of greater degradation rate of the fibrous fraction in feeds at low pH, thus increasing the production of microbial proteins and volatile fatty acids.
In conclusions the study shows that the microbial population and final fermentation products of the rumen are totally influenced by the diet provided to animals. The understanding of these processes may clearly elucidate how each of these microbial successions occurs. It will also allow maximizing the animal productive efficiency by manipulating diets, to generate practical recommendations for producers, for the optimization of nutrients use and maximization of the animal production with greater use of these feeds and, consequently, improving the incomes for rural properties.















