INTRODUCTION
Among the farm animals, goat was the first specie, domesticated so far. It was first reared by Iranian people dates back into 8000 1. Pakistan stands 3rd among the goat producing countries in the world after China and India 2. Goat is also recognized as poor men's cow in Pakistan, due to its high adoptability and acceptable production under the limited resources 3. It can be reared on marginal feeding materials and provides raw material for various valuable industries. Beside the food security, many farmers raised the goats as source of quick cash or for sacrifices on cultural events like Eid-ul-Adha 4. So, goat is kept for meat and rarely for milk purpose 5. Meat export sector it is playing a major role in livestock GDP. Demand of Pakistani meat has created a unique place in gulf due to its unique organic taste 6. Likely, livestock contribute 58.92 and 11.11% in the agriculture and national GDP respectively 7. Unfortunately, this GDP booster is also fronting many emerging challenges. By reason of urbanization, industrialization, growing food industry and dietary awareness among the masses, meat or meat product demand has been increased in the market too. In Pakistan meat consumption per person was 11.7Kg in 2000, it is projected to increase 32kg in 2018 and the demand of meat intake per capita expected to be upto the 47kg in 2020 8. But the traditional extensive goat farming system seems not capable enough to meet the ongoing demand and supply gape in the market. Extensive farming totally relies upon natural pastures (grasses shrubs or trees) on degradable land which is shrinking gradually 9. To surmount existing challenges of getting uninterrupted chevon supply, an improvement and intensification in reproductive performance of the goats by opting modern breeding tools is very much needed 10.
Breeding of animals is subjected to estrus synchronization in intensive goat farming system. Estrus synchronization technology provides great advantages like better estrus detection, wider application of artificial insemination 2, super ovulation and embryo transfer, induction of early puberty, increased number of kid crops and better management of pregnant goats 11. Numerous hormonal based stimulated (progestagens, gonadotropin, prostaglandins and melatonin) and bio-stimulation based (male effect, flushing diet and photoperiod control) estrus synchronization techniques have been effectively used by the workers. Different intravaginal devices that are impregnated with progesterone (P4) like controlled 2 internal drug release or CIDR containing natural P412 medroxyprogesterone acetate (MAP) sponges 13 and fluoroprogesterone acetate (FGA) sponges 14 are used to synchronize estrus in small ruminants. Medroxyprogesterone acetate and flugestone acetate are synthetic analogues of P4. Prostaglandin F2a (PGF2a) or its analogue cause regression of corpus luteum 15. When, P4 or its analogues are used in combination with PGF2a 16, they show better estrus induction 17.
In bio-stimulation methods, male effect is used for the synchronization of estrus in goats 18. The "male effect" is defined as the induction of estrus behavior and ovarian activity in female co-species under the influence of buck. Buck effect is one of the valuable tools that have been used to enhance the reproductive efficiency. It can be used to synchronize estrus in goats during breeding and non- breeding season as well the response to the "male effect" like velocity, serving capacity, intensity of stimulation of buck and percentage of ovulating goat mainly depend upon physiological, environmental, social and genetic factors 19. It has been studied that efficacy of estrus and fertility can be improved when male effect is used in combination with PGF2a and progestogens 20. Traditionally goats are kept isolated at least 45 days prior to buck introduction 21. After the period of isolation, the sudden exposure of male may induce LH surge and ovulation in the females 18. Studies have revealed that in goats and ewes LH surge normally occurs between 1-3 days after buck and ram exposure and ovulation occurs 22hrs after LH surge 22. For induction of pre-ovulatory LH peak and ovulation an intensive sexual behavior of buck is required. The intensity of sexual interaction mainly depends upon the presence of sexually experienced bucks 23. Buck effect has been used to induce estrus to get uniform kid crop during the non-breeding season 24. One of the benefits to stimulate estrus is that a large number of goats can be breed in a short period of time. Goats can be breed at the suitable time when their demand is high in market. With the growing demand of goat meat farmers need cost effective methods to produce kids during the period of low production. The "Buck effect" is a simple, cheap and easy to apply strategy to induce estrus in goat. Keeping in the view the dire need of effective goat farming techniques in country, present study was designed to investigate the effects of buck on reproductive performance of goats synchronized with our locally prepared medroxyprogesterone acetate (MAP) sponges.
MATERIALS AND METHODS
Medroxyprogesterone acetate (MAP) sponge preparation. Polyurethane foam was used to prepare sponges by Robinson's method 25, tied with polyester thread and washed with ethanol to remove debris. Stalk solution containing 60mg medroxyprogesterone acetate/ 3ml of ethanol was prepared and pour on each sponge. Sponges were suspended overnight under fume hood at room temperature. Medroxyprogesterone acetate (60mg) was retained inside the sponge after ethanol evaporation at room temperature. Completely dried sponges were dusted with penicillin, streptomycin powder and subjected to ultraviolet light exposure for sterilization.
Experimental site/farm. The present study was carried out during November to January, at Animal Farm, Nuclear Institute for Agriculture and Biology (NIAB), Jhang road, Faisalabad, Pakistan (31°23'43.9"N 73°02'12.3"E). All animal experimentation protocols will be approved by Animal Care and Use Committee of Nuclear Institute for Agriculture and Biology,Pakistan.
Animal selection. Twenty-eight multiparous, non-pregnant, goats (Dwarf x Beetal) were selected randomly during low breeding season from Novemmber to December, 2017. All were clinically healthy having body condition score equal or more than 2.5 (BCS 1-5). Four sexually active, healthy bucks were managed for breeding in Synchronized groups.
Animal feeding and health management. Barseem (Trifolium alexandrinum) fodder was offered to the goats, and they were also allowed to graze on seasonal grasses freely. Concentrate feed mixed (500g/animal/day) with wheat straw was offered daily before grazing. Water and mineral licks were provided ad libtum. For deworming Oxyclozanide 3.0% W/V, Levamisole Hydrochloride 1.5% W/V, Cobalt sulphate 0.382% W/V drench mixture (Levo Power drench® *M. S, Star, Pakistan) was used. Ivermectin (Bremamectin® 1ml/50kg, Bremer® Germany) was administered subcutaneously for tick's removal. Enterotoxaemia vaccine was used as prevention as per local vaccination schedule.
Estrus synchronization and buck exposure in goats. Refer to the figure (1.0, a & b), prior to the experiment goats were kept isolated from buck for 2 month the distance was more than 2km. Goats were divided in to two groups viz. buck exposed and buck isolated group. In buck isolated group (n=13) goats were selected. They were remained isolated from bucks (sight, smell) to avoid any pheromone interaction. Goats were kept in a shed more than 2 km away from the buck's pen. While in buck exposed group 15 goats were kept along with bucks were soon after the insertion of sponges. Goats were free to move as chemical communication took place between goats and bucks via pheromones interaction Sponges were inserted into the cranial part of vagina of each goat through paraffin oil lubricated speculum, with minimize resistance. A sterilized wooden rod was used to push the sponge through speculum. Rod and speculum were washed each time with povidine iodine (Pyodine®) solution for disinfection. Sponges were inserted for 15 day and after the sponge withdrawal an injection of PGF2a (d-cloprostinol; Synchromate® @37.5 µg/0.5ml intramuscular) was administered. After the removal of MAP sponge in buck isolated and buck exposed groups were mixed together and estrus detection was carried out. Estrus was detected with interval of 6hrs till 72hrs post sponges' removal. Estrus was confirmed by observing standing behavior of goat in front of buck. After the confirmation of standing estrus, estrus signs (bleating, tail waving, micturition, restlessness, off feeding, homosexual behavior, male seeking behavior, vaginal mucus secretion, tumefaction of vulva, and redness of vaginal mucus membrane) of the goats were observed. Natural mating was allowed in both groups.
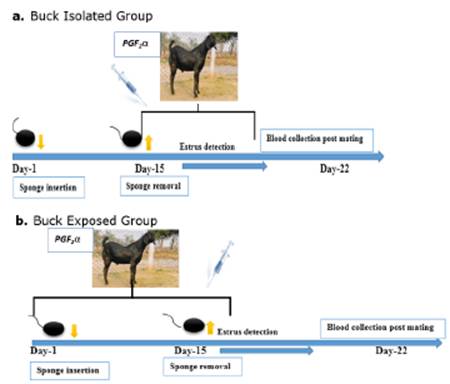
Figure 1 a. Isolated group (goats were kept isolated during the sponge treatment days); b. Buck Exposed group (goats were kept in continuous exposure with buck during the sponge treatment day.
Blood Sampling for Progesterone Analysis. Blood sampling was carried out on day 1, day 7, day 15, day 17, and day 27 of experiment for progesterone profile analysis for the detection of Ovulation rate and conception rate. On day 22 post mating blood sample was collected for early pregnancy diagnosis. Sterile glass tubes containing blood sample sssswere subjected at slanting position for the separation of serum. Serum was harvested in silicon tubes. That was subjected to centrifuge at 3000rpm for 15 minutes. Supernatant (clear serum) was extracted and kept at -20°C till for solid phase radioimmunoassay (RIA).
Progesterone Radioimmunoassay. Serum samples were analyzed for P4 by using solid phase radioimmunoassay kit (Beckman counter; Lot# IMI 188 & 4015B). The kit was supplied with the anti-progesterone antibody tubes, quality control, calibrators (standards) and iodinated progesterone (I-125 also known as tracer). The kit was provided with standards 0, 0.12, 0.55, 2.0, 8.7 and 49.0 ng/ml. After checking that all assay items (standards, samples, tracers & antibody coated tubes) are at room temperature, anti-progesterone antibody coated tubes were labeled for standards, quality control and for unknown serum samples in duplicate. Two TC (total count) labeled propylene tubes were also included in the assay. A 50 |il of each of the standards, quality control and serum were loaded into the correspondingly labeled tubes. After that with the help of dispenser, 500|il of tracer was dispensed in all tubes followed by incubation at 23-25oc along with horizontal shaking (150 rpm) for 1h. After that all tubes of the assay excluding TC were decanted and allowed to drain on absorbent paper till complete drying. Then all assay tubes were placed in a gamma counter (videogamma Rack, I'acn accessorio nuclear Italy) for radioactivity measurement. The standard curve was a logit-log graph, in which percent binding of standards relative to the references was drawn on vertical axis and concentration of standards on horizontal axis. The P4 concentration of unknown serum samples were measured by interposing from the standard curve. The highest percent binding (%B) of all assays ranged from 41%-53%. According to the specifications supplied with kit, the sensitivity of the assay was 0.05 ng/ml and the inter-assay and intra- assay coefficient of variation were 7.2 and 6.5% respectively (<10%).
Ovulation Rate. The goat showing P4 level > 1ng/ml on day 27 26 post sponge insertion were assumed as ovulation bearer. Ovulation rate was determined by dividing the number of goats ovulated to the total number of goats showing estrus signs.
Conception Rate. On the day 22 post-mating the does showing P4 level above 1ng/ml were considered as conceived 27. Conception rate was determined by dividing the number of goats conceived to the total number of goats being mated in the group.
Pregnancy Diagnosis through Ultrasonography. On day-35 post mating ultrasonography was performed for the pregnancy diagnosis. Goats were manually restrained in a standing position. A real time B-mode machine (Honda HS-1500) equipped with a 5.5-7.5MHz linear-array transducer was used. Carboxymethyl cellulose gel was applied on transducer and placed on hairless inguinal region across the abdomen cranial to the pelvic brim. After locating urinary bladder transducer was moved toward the uterus for detection of fetus or concave carnales inside the uterus.
Statistical analysis. Observations regarding estrus response, ovulation rate, conception rate, and pregnancy rate were compared by applying chi-square test (Statistix®, version 8.1). The level of significance was assumed as p<0.05. P4 profile and estrus behavior were presented as graphs in Microsoft excel (Microsoft Excel® 2010).
RESULTS
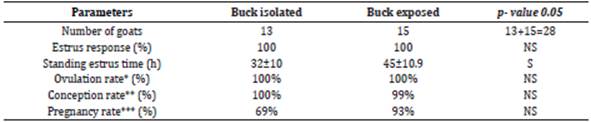
Goats felt comfortable with sponges, no change was observed in the behavior of goats after sponge insertion and removal. Sponge retention rate was 100%. Refer to the table 1, all goats showed estrus after sponge withdrawal in both groups (p>0.05). The intervals between sponge withdrawal and the onset of standing estrus were 32.3±10 h (18-51 h) and 45.2±10.9 h (27-51h) in buck isolated and exposed group respectively (p<0.05).
Table. 1 Effects of buck on the estrus response and subsequent fertility in the (Beetal x Dwarf) doe treated with MAP sponge.
*Based upon P4 concentration (1ng/ml) on day 10 post mating; **Based upon P4 concentration (1ng/ml) on day 22 post mating; ***Based upon ultrasonography on day 35 post mating
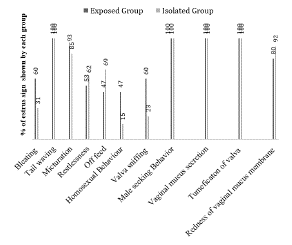
Primary estrus sign (stand to be mounted) and secondary estrus signs (bleating, tail wagging, and micturition, off feed, male seeking behavior, homosexual behavior, tumefaction of the vulva and redness of the vaginal membrane) were recorded and presented in the form of graph (Figure 2). Observations revealed that tail waving, male seeking behavior, mucus secretion and tumefaction of vulva were found in all estrus goats in both groups. Other signs (bleating, micturition, restlessness, off feed, homosexual behavior, and redness of vaginal mucus membrane) showed some variation but there was no significant difference in the frequencies of the aforesaid behaviors. Only vulva sniffing was observed significantly higher in buck exposed group (p<0.05).
According to the (Figure 3 and 4) of P4 concentrations in pregnant goats of both groups were not significantly different on day 0 (4.8±7 ng/ml vs 4.3±0.3ng/ml), while concentration of the P4 slightly gained momentum in exposed group on day 7 which was also not significant enough to say high (5.3± 7.2 ng/ml vs 3.3±2.8ng/ml). On day 15, in response to sponge withdrawal and PGF2co injection, concentration of P4 start falling along with estrus signs in both groups. P4 concentration on day 17 (1.28 ±0.4 ng/ml versus 1.7±0.907103 ng/ml; p>0.05) and day 27 (14.2±5.406421 ng/ml vs 14 ±8.5 ng/ml) were also recorded not significantly different (p<0.05).
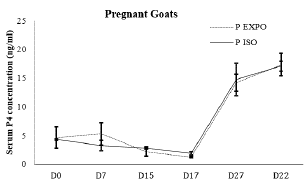
Figure 3 Pattern of progesterone profile in pregnant does of buck exposed and isolated group during treatment days
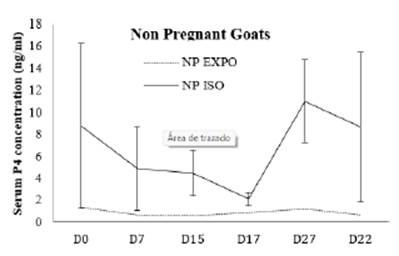
Figure 4 Pattern of progesterone profile in non- pregnant does of buck exposed and buck isolated group during treatment days.
Post estrus higher P4 (>1ng/ml) is refer to the ovulatory activity and this phenomenon was considered to predict ovulation in the goats. Based upon the serum P4 level on the day 27 post sponge insertion (10th day after mating) goats having level more than 1 ng/ml were marked as ovulated. In both groups all goats responded well, and the ovulation rates were 100% in buck isolated group and exposed group (p>0.05).
P4 concentration during the pregnancy remains higher than 1 ng/ml on day 22nd post mating in goats for sustaining pregnancy without showing any estrus activity. Based upon the P4 (>1ng/ml), conception rates in buck isolated and exposed groups were recorded as 100% and 93% accordingly (p>0.05). With the use of visual technique, ultrasonography on day 35 post mating, percentage of pregnant does were 69 vs. 93% in buck isolated group and exposed group respectively (Figure 5).
DISCUSSION
Our results reveal that estrus response was 100% in both groups in response to the MAP sponge. Efficiency of MAP sponge was equally good in both buck exposed and buck isolated group. Our results are matching with the results of previous studies conducted on does synchronize with commercially available medroxyprogesterone acetate sponge 28,29. Estrus response in our study was similar to the study conducted on Karakul ewes using MAP sponge in combination with eCG was better for artificial insemination 30. The time of standing estrus onset was significantly different in both groups. Overall goats in both groups showed standing estrus period between 16 and 76h after sponge withdrawal, with the highest incidence of standing estrus time occurring between 30 and 46 hr in both groups. The interval between sponge withdrawal and the onset of standing estrus were 32.3±10 h (18-51 h) and 45.2±10.9 h (27-51 h) in buck isolated and exposed group respectively (p<0.05).
The standing estrus onset time was significantly shorter in buck isolated group compared to the buck exposed group. The peak time for standing estrus was found 30hr after sponge removal in buck isolated group while in buck exposed group was 16hr later than the isolated group. This means that sudden exposure of the buck in previously isolated does have different estrus response compared to the continuous buck exposed does. The earlier estrus indication could be assigned to earlier production of estrogen from pre-ovulatory follicles in response of LH pulses because it is well known that the growth of pre-ovulatory follicles is LH pulsatility dependent 31. This result is in accordance with previous findings of Ungerfeld and Rubianes who reported that sudden introduction of ram cause 24 h earlier onset of standing estrus in previously isolated ewe synchronized with intravaginal MAP sponges 21. The difference in estrus onset interval between our findings and aforementioned research (16 vs. 24 h) may be due to specie difference (doe vs. ewe). It has been revealed that exposure of the ram into the pen of previously isolated ewes evoke significant increase in LH peak in most of the ewes in few minutes later 21. So that sudden exposure of the buck in a pen of previously isolated goats shorten the interval of standing estrus time due to LH pulses that cause production of estrogen consequently leading to the stimulation of follicular growth.
Onset of standing estrus was detected with the careful observation of estrus signs. The estrus signs are based on two types, primary and secondary signs 32. The primary signs are more authentic and well supported for the estrus indication of does. The best method adopted for the detection of estrus period was the careful observation of primary sign shown by the goats under the influence of the buck. However, the doe exhibit estrus response when they were on standing estrus such as male seeking, tail waggling when being introduced to buck and bleating was noticed when buck was absent. Vulva sniffing was observed significantly pronounced in buck exposed group compared to buck isolated group, the intensity of this parameter was in accordance with previous research who found vulva sniffing more intense in sexually active cows and termed as ovulation predictor behavior 33.
Aside from the primary sign, physical signs were considered as supportive sign for the accurate detection of estrus such as transparent mucus discharge, tumefaction of the vulva and redness of vaginal membrane, these signs were similar with the previous studies 33,34 which reported that the aforementioned primary and physical signs were present in cow at the time of estrus. Micturition, off feed, restlessness, homosexual behavior are secondary signs they were also in some of the female animal displaying estrus period. Secondary signs were less reliable because they were not present in all estrus animals, they may puzzle with the symptoms of slight illness. Our findings resemble with previous research 35, which showed that in buffalo homosexual behavior, frequent urination is present at the time of standing estrus, but these signs are not reliable of the confirmed estrus In our findings some of the primary and physical signs were present 100% in all goats of both group such as tail waggling, male seeking, mucus discharge and tumefaction of the vulva. Our results agree with previous findings 32 which demonstrate that aforesaid primary and physical signs are most authentic and reliable for the estrus detection in doe. Our results suggest to the farmer that they must observed carefully primary and physical sign that are 100% as discussed above, while detecting estrus in a herd of animals. These signs appear at the time of onset of standing estrus and disappear with the termination of estrus period. Detection of estrus is a useful parameter for natural mating, artificial insemination, anticipating ovulation time as well as parturition date.
In this work this was also found that continuous buck exposure during treatment days did not exert significant effect on P4 profile in cyclic does. P4 levels was found non-significantly higher trend on day 7 in buck exposed compared to buck isolated groups respectively (p>0.05). P4 prevents pulsatile release of the gonadotropin releasing hormone (GnRH) causing cessation of its production and secretion in ewe 36. The slight rise in P4 concentration on day 7 has been influenced by MAP to impede LH secretion hence earlier production of endogenous P4 in the observation period 37. On day 15 at the time of sponge withdrawal subcutaneous administration of PGF2a have luteolytic effect that hinder the function of corpus luteum leading to undetectable level of P4 has been reported 11,37 Lowest P4 level was observed on day of estrus showing minimum luteal activity. Other researchers revealed that animal showing P4 level more than 1ng/ ml not in estrus after sponge removal 38,39. Based upon present study it was suggested intravaginal MAP sponges and buck effect did not influence serum P4 profiles and concentrations. P4 levels were similar in the buck-exposed and isolated group. The results of current studies are in accordance with the previous studies by Omontese which reported that buck exposure did not exert significant effect on P4 profile of Sahel and Red Sokoto does 40.
Conception rates had no significant difference in both groups (buck exposed 93% and buck isolated group 100% respectively). The findings are again in accordance with the previous study 40 which reported that buck had no significant effect on the conception rate in Red Sokoto doe and Sahel doe which were treated with CIDR in combination with exposure of buck irrespective of the season. Conception rate was determined on day 22 post mating, animals showing P4 level more than 1ng/ml were considered pregnant 41. On day 35 post mating ultrasonography was performed to confirm pregnancy status in both groups. Based upon the results of ultrasonography, it was observed that 0% embryonic losses in buck exposed group while, 31% losses were seen in non-exposed group. Studies have reported that 6-42 % embryonic mortality happened during the first month of pregnancy in goats 42. Losses may be due to premature regression of corpus luteum produced in result of short-term buck exposure for mating in buck isolated group compared to buck exposed group 22. Whereas non-significant results of pregnancy rates were attained 93% in buck exposed group and 69% in buck isolated group.
On the basis of overall findings, it may be suggested that MAP sponge synchronization alone or in combination with bio stimulation (buck effect) have similar reproductive performance in term of pregnancy and P4 concentrations however onset of standing estrus time is influenced in does. Earlier estrus onset must be considered during the fixed time artificial insemination program.