Introduction
Adoption of Brachiaria forage grasses over the past 4 decades had a revolutionary impact on livestock productivity in the tropics (White et al. 2013). Both signalgrass [Brachiaria (now: Urochloa) decumbens cv. Basilisk, CIAT 606] and ruzigrass [Brachiaria (now: Urochloa) ruziziensis cv. Kennedy, CIAT 654] are grown on infertile acid soils that contain very low available phosphorus (P) levels, and are used for livestock production in the tropics (Miles et al. 2004).
A comparative study by Louw-Gaume et al. (2010a; 2010b), using signalgrass and ruzigrass, analyzed the role of morphological and physiological responses of roots, as plant mechanistic components to enhance P acquisition and P recycling within the plant. More specifically, plants of both grasses grown under low-P conditions had higher root biomass fractions and higher root tissue levels of acid phosphatases (APases) and phytases than plants grown under high-P conditions. Interestingly, root morphological traits of signalgrass were not responsive to variation in P supply, while lateral root growth in ruzigrass was significantly increased in plants grown at low P supply in hydroponic growth conditions.
Veneklaas et al. (2003) suggested that the key factor in plant-soil interactions might be rhizosphere chemistry, rather than root morphology. Mechanisms to increase inorganic P (Pi) availability in the rhizosphere include carboxylate exudation and APase secretion by plant roots (Gaume et al. 2001; Lambers et al. 2006; Neumann and Rõmheld 2012). The induction of APases is a general response of plants to Pi starvation and correlations between the intracellular and/or extracellular APase activity and cellular Pi status have been found (Vance etal. 2003; Nanamori et al. 2004).
Carboxylates enhance Pi release through the dissolution of calcium (Ca), iron (Fe) or aluminum (Al) phosphates. However, little in vivo evidence for P mobilization by carboxylates exists, except that most P-deficient plants release higher amounts than P-sufficient plants (Strõm et al. 2002). The organic acid anions most effective at mobilizing P in soils are, in descending order, tricarboxylate citrate and the dicarboxylates, oxalate and malate (Neumann and Rõmheld 2000; 2012). Carboxylate release also requires the counter release of a cation to maintain charge balance. In the case of P deficiency, the rhizosphere pH has been shown to decline concurrently with carboxylate release, suggesting a balancing role for proton (H+) efflux via H+-ATPases (Hinsinger et al. 2003; Neumann and Rõmheld 2012). Two other likely candidates in terms of counter ions are potassium (K) and magnesium (Mg). Increased K+ concentrations in root exudates suggest that carboxylate- and K+-effluxes are coupled (Ryan et al. 2001), while Zhu et al. (2005) reported the involvement of Mg2+ in P-limiting carboxylate release in white lupin. Benefits for P uptake resulting from the coupling of carboxylate release to K-efflux have been shown by Palomo et al. (2006) as rhizosphere alkalinization by K-citrate-enhanced P mobilization in a high P-fixing acid soil.
The objective of this study was to determine the differences in the release of root biochemical markers, i.e. carboxylates and APases, during the development of P deficiency in signalgrass and ruzigrass. Our hypothesis was that exudation rates of both biochemical markers of P deficiency will be augmented in P-deficient plants of both grasses, but the 2 grasses could differ qualitatively and quantitatively in their response, when grown for a short period of 21 days at low P supply. We used the hydroxyapatite pouch system in hydroponics (Sas et al. 2001) to simulate low P supply conditions of infertile tropical soils (Louw-Gaume et al. 2010a) and to investigate whether the release of carboxylates and APases from roots is part of a temporally coordinated and targeted response to P limitation in signalgrass and ruzigrass. In addition to these physiological responses and associated differences in plasticity, related mechanistic components such as exuded counter-ions for charge balance and tissue levels of carboxylates were investigated at weekly intervals for 3 weeks. Finally, as responses in leaf and root growth of Brachiaria grasses might differ at low P supply (Rao et al. 1996; Louw-Gaume et al. 2010a, 2010b), we also examined these morphological responses in order to obtain a whole-plant perspective that might contribute to understanding diversity in plant attributes for tolerance to low-P acid soils that exists in Brachiaria germplasm (Rao et al. 1998; Miles et al. 2004; Rao 2014).
Materials and Methods
Plant growth and harvests
The experimental protocol for the germination of seeds and growth of a tetraploid, apomictic signalgrass [Brachiaria (now: Urochloa) decumbens cv. Basilisk, CIAT 606] and a diploid sexual ruzigrass [Brachiaria (now: Urochloa) ruziziensis cv. Kennedy, CIAT 654] in nutrient solution at pH 5.5, using the hydroxyapatite (HAP)/dialysis pouch system, was reported by Louw-Gaume et al. (2010a). Seeds were surface-sterilized and germinated in the dark (25 °C) for 3 to 4 days on filter paper saturated with deionized water. Seedlings were grown for one week in sand culture (with nutrient supply in mg/kg of sand: 2.6 P, 2.5 N, 3.1 K, 1.0 Ca, 0.38 Mg, 0.38 S, 0.02 Zn, 0.03 Cu, 0.001 B, 0.001 Mo) in growth chambers with a day/night cycle of 12 h at 25 °C and 12 h at 18 °C, 60% relative humidity and a photon flux density of 250 umol/m2/sec. These conditions for early seedling growth were used since Brachiaria grasses do not display rapid early seedling growth level due to their small seed size. Selected seedlings of each grass with similar development were further grown in aerated nutrient solution (in mM: 0.25 NH4NO3, 0.53 KNO3, 0.75 Ca(NO3)2, 0.33 CaCh, 0.42 MgSO4, 0.17 NaCl, 0.01 FeNaEDTA; in uM: 30 H3BO3, 5 ZnSO4, 0.2 CuSO4, 10 MnCl2, 0.1 Na2MoO4) under the same controlled conditions. The hydroxyapatite/dialysis pouch system in hydroponics was used to induce high-P (5 g of hydroxyapatite) and low-P (1 g of hydroxyapatite) conditions (Louw-Gaume et al. 2010a). The current study included only the low-P treatment to further characterize physiological and biochemical responses of both grasses to low P supply. Plant responses were monitored at 3 time intervals, i.e. day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low P supply to one-week-old seedlings transferred to nutrient solution. This growth period was selected to focus on root-level mechanisms for P uptake during vegetative growth.
Phosphate release in control containers (n = 6) without plants was monitored and measured as 0.33 ± 0.02 uM Pi/d. The mean concentration of Pi on day 0 (day before introducing seedlings) was 1.00 ± 0.11 uM (n = 16), a Pi level that is in agreement with the value of 1 uM Pi used by Wenzl et al. (2003) to simulate Pi level in soil solutions of highly weathered acid soils. On a daily basis, the pH and Pi concentration of hydroponic solutions were measured, while the HAP-containing pouch was checked daily for potential leakage and visible evidence of bacterial growth. The complete nutrient solution, including the pouches, was renewed on days 8 and 15 (D8 and D15). As described before (Louw-Gaume et al. 2010a), each hydroponic tank contained 2 replicates of each grass and each replicate consisted of 3 plants. The number of replicates was 10 for each grass (that is, 30 plants in total). The experiment was repeated and the data from the second experiment are reported, since this experiment included all measurements. Similar results were observed in both experiments on biomass production, carboxylate composition and exudation rates for both grasses.
Three destructive harvests were performed following the collection of root exudates at D7, D14 and D21, starting at the same time of day for each harvest, as it has been reported that rhizosphere processes for P mobilization exhibit a temporal variability (Neumann and Rõmheld 2012). The dry matter (DM) per young seedling (n = 10) before inducing low-P treatment was slightly higher for ruzigrass than for signalgrass (37 vs. 32 mg DM). The shoot mass density of signalgrass seedlings was higher than for ruzigrass (0.16 vs. 0.13 g DM/g fresh biomass). The nutrient concentrations (% of dry weight) of the seedlings before low-P treatment were: 0.16 P, 0.19 S, 1.14 N and 44.5 C for the shoot tissue of signalgrass; and 0.23 P, 0.31 S, 4.29 N and 42.0 C for the shoot tissue of ruzigrass. For root tissue of the seedlings, the concentrations were: 0.07 P, 0.12 S, 1.14 N and 44.5 C in signalgrass; and 0.08 P, 0.13 S, 1.36 N and 39.3 C in ruzigrass. Plant material was dried for 4 d at 45 °C before DM determination. Leaf area was recorded with a leaf area meter (Li-COR Model 3100, Lincoln, USA). The complete root system was scanned and root length was analyzed using WinRHIZO V3.09b root imaging software (Regent Inc., Quebec, Canada). The relative growth rate was calculated for each harvest interval, according to the method of Hoffmann and Poorter (2002). Rates of leaf expansion and root elongation between harvests were calculated as the change in leaf area (in cm2) or root length (in m) per day for the three 7-day growth periods. For the determination of the plant P, K and Mg concentrations, dried and milled plant material was incinerated at 550 °C, followed by solubilization in 65% HNO3 and analysis with ICP-emission spectroscopy (Louw-Gaume et al. 2010b).
Collection of root exudates andpH measurement in CaCh traps
At each sampling plants were removed from hydroponic containers and root systems were washed twice in 0.1 mM CaCl2 solution (pH 5.5, adjusted with HCl) to eliminate possible interference from remaining nutrients close to root systems during the exudation steps. Great care was taken when handling root systems to avoid tissue damage. The first exudation step was performed for 6 h in aerated 0.1 mM CaCl2 (pH 5.5) solution containing 0.01% (v/v) protease inhibitor cocktail (Sigma, P2714) under the same growth chamber conditions as for plant growth. The second step was carried out for 1 h at 4 oC in 0.1 mM NaCl (pH 5.5, adjusted with HCl) with the same inhibitor cocktail. Exudation volumes were adjusted at each harvest time to compensate for different plant sizes. For example, at D7, the exudation volume was 30 ml per bunch of 3 plants for both grasses and 80 ml and 110 ml for signalgrass and ruzigrass, respectively, at D21. The pH was measured at the end of the 6-h period in the CaCh solutions. Exudates were centrifuged at low speed (4 oC) for 3 min, filtered through 0.2-um syringe filters and stored at -80 oC until assayed. These steps were in line with recommendations by Gaume et al. (2001) and Neumann and Rõmheld (2000).
Carboxylate extraction and determination
The roots were washed with de-ionized water and blotted dry with paper towels, frozen in liquid nitrogen and stored at -80 oC until extraction. The method of Zindler-Frank et al. (2001) was slightly modified and soluble oxalate was extracted by grinding frozen leaf and root material in warm (50 oC) deionized water, followed by heating at 80 oC for 30 min, bench-top centrifugation, filtration through 0.2-um syringe filters and acidification with HCl to pH 3-4. These extracts were also used for the determination of glycolate.
The carboxylates in vacuum-concentrated CaCl2 solutions were analyzed by ion chromatography (Dionex DX 500 System, Dionex Corporation, USA). An Ion Pac AS10 column, in combination with suppressed conductivity, was used and the eluent was 50 mM NaOH with a flow rate of 1 ml/min. The exudate samples were dried and the pellets re-suspended in nanopure water prior to injection. The identification of carboxylates was confirmed by spiking with standards and carboxylate release rates were expressed per unit of root length, i.e. nmol/m/h.
Effluxes of H + , K + , Mg 2+ and NO 3 ‾
At each harvest time, the pH of CaCl2-exudate solutions increased from 5.5 to values above 6 for both grasses over the 6-h exudation period. These increases were converted into proton equivalents and expressed as the relative change in protons. Efflux rates of K+ and Mg2+ were determined by analyzing the K and Mg concentrations in the CaCl2 solutions with ICP-emission spectroscopy. The CaCl2 solutions were also analyzed for the presence of nitrate (NO3 ~) using a flow injection analyzer (SKALAR San++ System, Netherlands). The relative change in protons and efflux rates of K+, Mg2+ and NO3~ were expressed per unit of root length, i.e. umol protons, K+, Mg2+/m/h and nmol NO3 ~/m/h.
Acid phosphatase and phytase activity
Acid phosphatase activities detected in the CaCl2 and NaCl solutions were grouped as secreted APases (sAPases) and cell-wall-associated APases (cwAPases), respectively. Root exudate solutions were concentrated with centrifugal filters (Amicon Ultra-15, Millipore, USA) for the detection of phytase activity. The activities of acid phosphomonoesterases and phytases were determined as described by Louw-Gaume et al. (2010b). Enzyme activities were expressed as enzyme units (U) per unit root length, where 1 U releases 1 umol Pi/min.
Statistical analysis
The Welch two sample t-test was used to determine differences between species and between harvest intervals (R Core Team 2014).
Results
Biomass production and plant P concentrations
The total biomass production increased between sequential harvests for both grasses, but ruzigrass produced more biomass at each harvest time (Figure 1A).
Figure 1B shows that ruzigrass could not maintain its relative rate of biomass production after D14 (14 days after inducing low-P treatment) and the relative growth rate declined by 30%, to a level similar to that maintained by signalgrass throughout. Rate of leaf expansion increased strongly between D7 and D14, with a smaller increase in rate from D14 to D21, while rate of root elongation for ruzigrass was much greater between D14 and D21 than for the other periods (Figures 1C and 1D, respectively). Root diameter was not affected by decreasing plant-P concentrations, although signalgrass had thinner roots at each harvest time (results not shown).
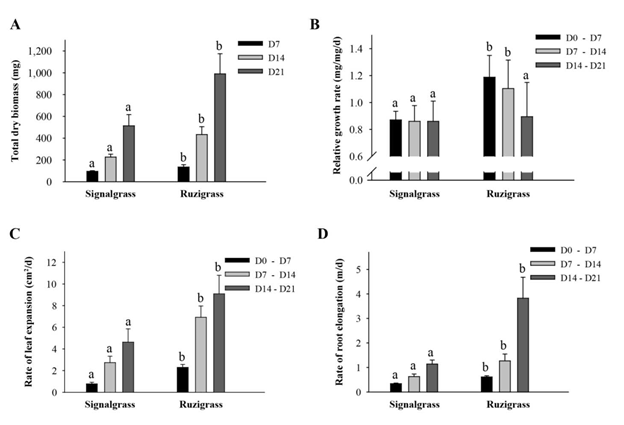
Figure 1 Morphological attributes of signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low-P treatment under hydroponic conditions. (A) Total dry biomass (DM). (B) Relative growth rate (mg/mg/d) for each of the 3 harvest intervals, i.e. D0-D7 (first harvest interval), D7-D14 (second harvest interval) and D14-D21 (third harvest interval), where D0 refers to the start of the experiment and the day on which young seedlings were prepared for experimental use. (C) Rate of leaf expansion (cm2/d). (D) Rate of root elongation (m/d). Means for a specific harvest time or harvest interval with different letters indicate significant differences between grasses (P<0.05).
Plant-P concentrations in both grasses declined with age with a greater reduction for ruzigrass than for signalgrass (Figure 2). P concentration in ruzigrass at D7 was much greater than for signalgrass (3.8 vs. 2.1 mg/g DM) but levels were similar for both grasses at subsequent harvests, reaching about 1.0 mg P/g DM at D21.
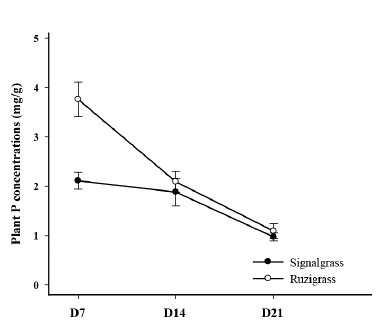
Figure 2 Plant-P concentrations of signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21), expressed as mg P/g DM, after inducing low-P treatment under hydroponic conditions. Vertical bars represent ± s.e. (n = 30).
Carboxylates in root exudates and in tissues
Figure 3A shows exudation rates and composition of organic acid anions, i.e. acetate, glycolate, formate, lactate (monocarboxylates) and oxalate (dicarboxylate). Citrate (tricarboxylate) and malate (dicarboxylate) could not be detected in the root exudates of either grass. The combined exudation rates of all carboxylates for ruzigrass were 115% greater than those for signalgrass at D7, 240% greater at D14 and only 55% greater at D21. The temporal patterns of monocarboxylate exudation did not differ between grasses, with rates decreasing after D7 (Figure 3B), but then increasing slightly between D14 and D21. In contrast, patterns of oxalate exudation differed between grasses (Figure 3C) with rate increasing throughout for signalgrass but peaking at D14 for ruzigrass. Final levels at D21 were similar for both grasses.
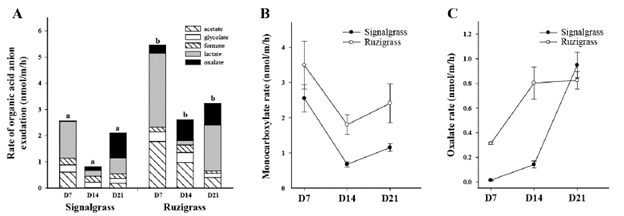
Figure 3 Root exudation rates (nmol/m/h) of carboxylates by signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low-P treatment under hydroponic conditions. (A) Total root exudation rates and carboxylate composition. Different letters indicate significant differences between grasses for total root exudation rate at a specific harvest. (B) Rates of monocarboxylate root exudation, including acetate, glycolate, formate and lactate. (C) Rates of oxalate root exudation.
The carboxylate composition of root exudates changed over time for both grasses (Figure 3A). The oxalate fraction at D7 and D14 was greater for ruzigrass than for signalgrass (5 and 31% vs. 1 and 17%, respectively). By D21 the level in signal grass had increased to 45%, while the level in ruzigrass remained at 31%. Patterns of lactate exudation were similar in both grasses, being high at both D7 and D21 with very low levels at D14. At D21 signalgrass had a higher oxalate:lactate ratio than ruzigrass (1.7 vs. 0.5). For both grasses, the temporal patterns for glycolate and formate fractions were similar; absolute levels of exudation did not vary over time as much as levels of acetate, oxalate and lactate but the percentages of total exudation fluctuated because of changes in the other components.
Tissue concentrations of soluble oxalate and glycolate are shown in Table 1. For each grass, leaf and root oxalate concentrations did not change between D7 and D14. At these harvest times, signalgrass had greater leaf:root oxalate ratios than ruzigrass due to oxalate concentrations in signalgrass being higher in leaves and lower in roots than those of signalgrass. Leaf oxalate concentrations in signalgrass decreased after D14, while for ruzigrass, both leaf and root oxalate concentrations declined. Glycolate concentrations were up to 30 times those of oxalate in both grasses. In addition, leaf glycolate concentrations showed moderate temporal variation, with lowest values at D14, while root glycolate levels changed in a similar way to oxalate levels in each grass.
Table 1 Tissue concentrations (nmol/g fresh mass) and leaf:root ratios of oxalate and glycolate for signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low-P treatment under hydroponic conditions.
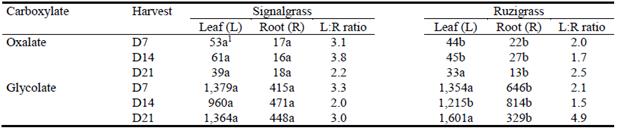
1Within rows and plant parts, values followed by different letters are different (P<0.05).
Changes in proton equivalents and efflux rates of K + , Mg 2+ andNO 3 ~and root concentrations of K andMg
The relative change in proton equivalents (Figure 4A) was greater for signalgrass than for ruzigrass at D7, but grasses did not differ at D14 and D21. For both grasses the lowest values were recorded at D21 (i.e. pH increased to a lesser extent from the value of 5.5 after D14). Rates of efflux of NO3" (Figure 4B), K+ (Figure 4C) and Mg2+ (Figure 4D) generally increased with time, while root concentrations of K and Mg declined over time (Figures 4E and 4F).
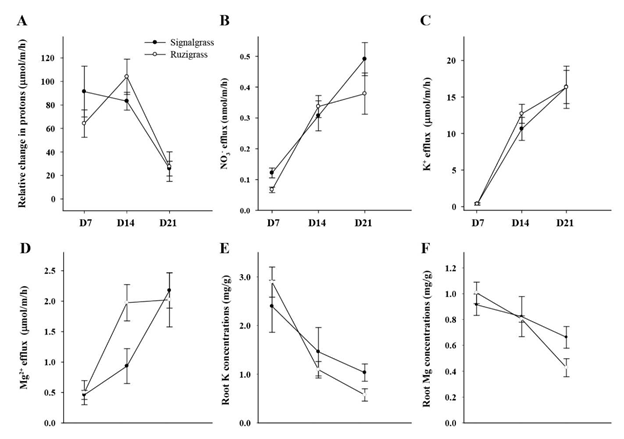
Figure 4 Relative change in proton equivalents (umol/m/h) and efflux rates of NO3"" (nmol/m/h), K+ (umol/m/h) and Mg2+ (umol/m/h) and K and Mg concentrations (mg/g) in roots of signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low-P treatment under hydroponic conditions. (A) Change in pH (from pH 5.5) expressed as relative change in proton equivalents. (B) Rate of NO3_-effux. (C) Rate of K+-effux. (D) Rate of Mg2+-efflux. (E) Root K concentrations. (F) Root Mg concentrations.
Temporal patterns of APase secretion and phytase proportion
Rates of secretion of sAPases were similar in both grasses at D7 and D21, but ruzigrass had a much higher secretion rate at D14 than signalgrass (Figure 5A). For both grasses, cwAPase release rates increased only after D14, by 2-fold in signalgrass and 6-fold in ruzigrass (Figure 5B). Compared with sAPases at D21, rates of cwAPases were 3-fold higher in signalgrass and 10-fold higher in ruzigrass. Extracellular phytases could be detected only at D21 in both the CaCl2- and NaCl-collections. Phytase proportions (as a percentage of the total APase pool) were low for both grasses, but were slightly higher in the cwAPase pool than in the sAPase pool.
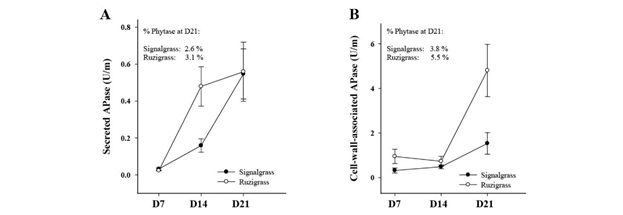
Figure 5 Root exudation of APases (U/m) by signalgrass and ruzigrass at day 7 (D7), day 14 (D14) and day 21 (D21) after inducing low-P treatment under hydroponic conditions. (A) Rate of secreted APases collected in CaCl2 solution. (B) Rate of cell-wall-associated APases collected in NaCl solution. Activity of extracellular phytase was detected only at D21 and its percentage of the total pool of APases is indicated in the left upper corner of each graph.
Discussion
Low P supply reduced biomass production and leaf expansion in ruzigrass
In agreement with earlier findings (Louw-Gaume et al. 2010a), ruzigrass was a faster-growing grass and produced more biomass than signalgrass at low P supply during this short experimental period of 21 days that focused on plant mechanisms and associated plasticity for P uptake during early vegetative growth. However, the growth of ruzigrass was compromised during the development of P deficiency; while ruzigrass grew very fast initially, it could not maintain its relative growth rate and strong leaf expansion after D14. In addition, plant-P concentrations declined after D14 to below 2.0 mg P/g DM, indicating that ruzigrass started to economize on Pi. Veneklaas et al. (2012) suggested that the reduction in growth is not a direct consequence of low shoot-P status, but of signaling events that can be genetically controlled. Lambers et al. (2008) also reported that roots sense that nutrients such as N and P are limiting well before leaves experience deficiency symptoms, indicating that shoot growth is regulated in a feed-forward manner.
Critical shoot-P concentrations in Brachiaria grasses are around 1.0 mg P/g DM (Rao 2001). For ruzigrass, the key factor responsible for high P uptake and high initial P concentrations might be a faster growth rate as suggested by Lambers and Poorter (2004). In contrast, signalgrass had lower P uptake and P concentration in tissue initially, resulting in lower growth rates. This balanced growth rate may ensure that nutrient demand does not exceed its supply.
Release of oxalate and APase are linked to decreasing plant-P concentrations
Our results suggest that oxalate and APases are involved in the P-nutrition of both Brachiaria grasses as temporal associations between decreases in plant-P concentrations and increases in the exudation of these biochemical attributes were evident. The release of both components might also form part of a coordinated adaptive strategy and functional synergy between oxalate and APases, which could improve acquisition of P in low-P acid soils as carboxylates can enhance the solubility of not only inorganic P, but also organic soil-P forms, which are subsequently hydrolyzed by phosphatases (Vance et al. 2003; Jones et al. 2004; Playsted et al. 2006).
The increase in exudation of oxalate and sAPases by roots could be associated with decreases in P concentrations in each grass. These traits increased during early growth, together with the first decline in plant-P concentrations in ruzigrass, supporting as well a higher growth demand for P in this grass. While rate of exudation of carboxylate and sAPase by ruzigrass peaked at D14, secretion of cwAPase increased sharply during the next 7 days, when plant-P concentrations declined further and biomass production would have been compromised. It is important to emphasize that, although root exudation responses of ruzigrass after D14 appeared to level off, root elongation increased strongly after D14. Thus, if root growth was stimulated while exudation rates were maintained during P-limited growth, the key factor to consider was total below-ground output of carboxylates, which might be higher for ruzigrass. Louw-Gaume et al. (2010a) also showed that lateral root growth was stimulated in ruzigrass only when grown at low P supply. Interestingly, Hütsch et al. (2002) reported that cultivar differences in total amounts of root-released C could be attributed to root length.
Furthermore, it appears that root morphological plasticity in ruzigrass is associated with a high level of root physiological plasticity as evident from the strong induction of cwAPases by plant P concentrations below 2 mg P/g DM. This finding also suggests a dependence on a critical threshold of Pi depletion as a signal for enzymatic cwAPase induction (Jain et al. 2007). In white lupin, secretory APases were produced not only by tap root epidermal cells, but also in the cell walls and intercellular spaces of lateral roots. Such apoplastic phosphatases are protected from inactivation by various soil processes, but effectiveness depends on the presence of soluble organophosphates in soil solution (Neumann and Rõmheld 2007; 2012). Although the enzymatic hydrolysis of root-secretory phosphatase is limited by the low solubility of organic P forms in soils (Neumann and Rõmheld 2012), higher phosphatase activities in the rhizosphere have been reported to contribute to the depletion of organic P from Oxisols containing very low available P (George et al. 2006). In signalgrass, exudation responses of all 3 biochemical markers for P limitation, i.e. oxalate and both groups of APases, were temporally coordinated and increased only after D14.
Acquisition of P from phytate by phytases could potentially provide plants with an alternative organic P source (Richardson et al. 2005). Louw-Gaume et al. (2010b) reported higher root tissue levels of APases and phytases for plants grown under low-P conditions with phytase proportions representing less than 1% of the total APase pool in root tissue, while the present study found higher phytase proportions in both the sAPase and cwAPase pools (2 and 5%, respectively). Interestingly, the grasses we studied did not differ with regard to phytase fractions in either study. Our results are at variance with the findings by Li et al. (1997), who reported high levels of phytase secretion in P-deficient B. decumbens plants. The experimental system used in simulating low-P supply conditions in the hydroponics growth medium might explain these differences. Our results support the observations of Hayes et al. (1999), who reported that phytase activity constituted only a small component (less than 5%) of the total APase activity in various plants.
Oxalate exudation may enhance P acquisition in acid tropical soils
Oxalate exudation in response to P deficiency has been reported in sugarbeet (Gerke et al. 2000), soybean (Dong et al. 2004), rice (Hoffland et al. 2006) and Banksia species (Denton et al. 2007) and our results for both Brachiaria grasses are consistent with these observations. Pentanedioic acid and oxalic acid were also dominant exuded organic acids in P-deficient elephantgrass (Pennisetum purpureum), another tropical forage grass (Shen et al. 2001). Dong et al. (2004) also noted that exudation of oxalate rather than other carboxylates may present higher physiological efficiency, as less C and energy are consumed during exudation.
Hydroponic experiments provide only indirect evidence and the functional significance of carboxylate exudation in a real soil environment remains unknown (Jones et al. 2004; Neumann and Rõmheld 2012). Observed exudation rates cannot be compared with those of leguminous plants as their carboxylate effluxes are 10 to 50 times higher than for graminaceous species (Gerke et al. 2000). In soils with low P-availability, competition by carboxylates for P-sorption sites might be of greater significance than P-desorption mechanisms, which require high concentrations of carboxylates such as citrate and oxalate. Huguenin-Elie et al. (2003), using a modeling approach, showed that low release rates of citrate could account for 90% of the P uptake of rice grown under aerobic conditions. Furthermore, average values integrated over the whole root system can be misleading and may result in erroneous conclusions about nutrient relationships in the rhizosphere, due to spatial variability of exudation along the root axis (Neumann and Rõmheld 2000; 2012).
Fox and Comerford (1992) suggested that the cumulative oxalate loading rate contributes to the solubilization of large amounts of P on an annual basis and this might be relevant for the survival of the grasses used in our study. Furthermore, the effectiveness of oxalate was shown in both calcareous and acid soils treated with monocalcium phosphate and phosphate rock (Fox and Cromerfold 1992; Strõm et al. 2002), suggesting that oxalate exudation by signalgrass and ruzigrass might have significance for enhanced P-acquisition in acid soils. Application of rock phosphates to acid soils has been suggested (Fardeau and Zapata 2002) and their suitability as P fertilizer for signalgrass has been demonstrated (Lopes et al. 1991). Araújo et al. (2003) also reported greater importance for the acid-soluble P fraction than for the NaOH-extractable fraction in a pot experiment using B. decumbens. As signalgrass is better adapted to and more persistent on infertile acid soils that contain very low available P than ruzigrass (Miles et al. 2004; Rao 2014), signalgrass might have a selective ecophysio-logical advantage over the long term due to the dominance of oxalate plus its slower and more balanced growth rate and associated implications for higher plant carbon (C) use efficiency (Louw-Gaume et al. 2010b). Leaf oxalate concentrations were also higher for signalgrass, consistent with higher oxalate levels reported for slower-growing plants (Libert and Franceschi 1987).
Although lactate appears to be commonly exuded by plant species that are adapted to acid soils (Tyler and Strõm 1995), the finding that lactate was the dominant exuded carboxylate in ruzigrass at D21 was unexpected, as the presence of lactate has also been linked to detoxification that could be associated with cytoplasmic acidosis (Neumann and Rõmheld 2000). It is possible that, despite its high biomass production, high P uptake and high exudation rates of biochemical traits important for P-mobilization in acid soils, ruzigrass might start to experience metabolic complications in maintaining cellular Pi homeostasis over a longer growth period (Veneklaas et al. 2012). In addition, C-costs related to exudation (Dilkes et al. 2004) might have been substantial in ruzigrass, as faster-growing grasses deposit more C than species adapted to infertile soils (Warembourg et al. 2003).
The higher rate of formate exudation in signalgrass was also interesting, as Tanaka et al. (1995) suggested that formate could solubilize Fe-P forms due to its strong reducing capacity, based on observations of increased formate secretion in P-deficient Arachis hypogaea.Dinkelaker et al. (1995) also proposed that increased reductive capacity in roots may be another P-adaptive response.
Oxalate exudation might be an important strategy for Al resistance, as Al-toxicity and P-deficiency co-exist in acid soils and both are major constraints for productivity of Brachiaria pastures (Miles et al. 2004). Carboxylate exudation could not be linked to external Al detoxification in either grass (Wenzl et al. 2001), but a low-P background might have obscured responses (Liao et al. 2006). Interestingly, phytosiderophore-mediated iron release from goethite is also enhanced by oxalate (Marschner et al. 2011) and thus, oxalate might also be important for Fe uptake from iron oxides in both Brachiaria grasses.
Are Mg 2+ ions involved in charge balance during oxalate exudation?
Our observations reiterate that interpretation of pH changes in the rhizosphere should be considered with caution (Hinsinger et al. 2003; Neumann and Rõmheld 2012). The greater pH of CaCl2-containing root exudates may be attributed to lower Ca2+ uptake (versus Cl~) (Hinsinger et al. 2003). In B. dictyoneuraHylander and Ae (1999) also reported an increase in the rhizosphere pH due to higher amounts of basic cations and proton neutralization. However, the pH of nutrient solutions with growing plants declined over time and ruzigrass showed greater capacity to lower the pH (observed in pre-experiments), so we adopted the practice of growing both grasses in the same hydroponic container to eliminate interferences from the addition of KOH that was used for pH control. Proton release has also been linked to differential cation/anion uptake (Hinsinger et al. 2003), consistent with the report by Logan et al. (2000), who found that plant-induced acidity by B. humidicola and B. brizantha was not due to low P-availability, but to adequate supply of nutrients for growth.
Our study focused on the most likely counter-cation candidates to accompany carboxylate efflux (Ryan et al. 2001; Zhu et al. 2005). Interest in K+-efflux and root-K levels also stems from the finding that the K or sodium salt of oxalate is predominantly found in grasses (Jones and Ford 1971). The two grasses did not differ in the pattern of K+-efflux, which increased strongly after D7. Marschner et al. (1997) reported that K+ functions in charge balance, especially in NO3 ~-fed plants, participating as well in translocation of carboxylates and soluble sugars. Interestingly, an increase in NO3 ~-efflux after D14 was observed only in signalgrass, supporting higher NO3 ~-efflux rates as reported in slow-growing plants (Nagel and Lambers 2002). Root-K concentrations decreased for both grasses as reported during P deficiency in white lupin (Sas et al. 2002) and the Brachiaria hybrid cv. Mulato (Watanabe et al. 2006).
Despite these uncertainties for H+ and K+, Mg2+ appears to be a counter-ion for oxalate efflux as its efflux pattern corresponded well with the release curves of oxalate in both species. Zhu et al. (2005) reported that Mg2+ was involved in carboxylate release of white lupin during P deficiency. Increases in Mg2+-efflux by roots also corresponded in a timely manner with decreases in root concentrations of Mg in each grass.
Another consideration is that cation-efflux rates were higher than those of carboxylates. Deficiency of P enhances membrane leakiness (Neumann and Rõmheld 2007), suggesting that the likelihood of higher nonspecific efflux during P limitation cannot be excluded.
Glycolate might be an oxalate precursor
As expected, monocarboxylate exudation could not be related to the plant-P status in the current study, but our results on monocarboxylate composition and exudation patterns could have significance for C utilization. Oxalate can be formed from photorespiratory glyoxylate via glycolate, catalyzed by glycolate oxidase (Franceschi and Nakata 2005). In both grasses we used glycolate might be an oxalate precursor, as leaf glycolate levels were significantly higher than those of oxalate and, in addition, leaf oxalate levels decreased after D14, while leaf glycolate levels increased. Interestingly, Ueno et al. (2005) studied 28 C4 grasses (ruzigrass not included) and found activity of glycolate oxidase was greatest in B. brizantha and B. decumbens.
While oxalate exudation increased after D14 in signalgrass and also ruzigrass (when increases in root elongation are considered), leaf oxalate concentrations decreased for both grasses, suggesting that leaves might be the site of oxalate biosynthesis, as reported by Ji and Peng (2005). Root levels of oxalate and glycolate did not change over time in signalgrass, but both decreased strongly in P-deficient ruzigrass plants. Carboxylate efflux has been correlated with intracellular root concentrations, but Ryan et al. (2001) pointed out that membrane processes appear to be the key step for efflux.
Our results support the notion that increased carboxylate biosynthesis in plants is a physiological alteration associated with the preferential root exudation of carboxylates with highest efficiency in P mobilization under conditions of P limitation (Neumann and Rõmheld 2007).
Conclusions
The experimental approach used in this study highlights the importance of adopting an eco-physiological perspective to understand developmental, physiological and biochemical aspects of adaptation to low-P stress in Brachiaria grasses. Furthermore, a comparison of species differences in adaptation to limiting P supply became feasible by studying, simultaneously, temporal responses of: (i) whole-plant growth together with variations in both root and leaf attributes; and (ii) root-induced changes in the rhizosphere that determine nutrient availability and influence plant growth. Results from this study indicate that growth may be faster for ruzigrass than for signalgrass during early establishment in low-P soils but ruzigrass may demand higher P supply to sustain its higher growth rate. Further research is needed on soil-grown plants of both grasses to characterize changes in rhizosphere induced by exudation of organic acids and phosphatases from roots.














