Introduction
Concern about the invasive potential of trees in the Leucaena genus has led to a desire to produce and plant sterile hybrids or varieties. Some of the interspecific crosses between diploid and tetraploid species have resulted in sterile or low-fertility triploid offspring (Sorensson and Brewbaker 1994; ). This provides opportunities to breed for sterility as well as providing suitable characteristics for a range of environments and end-product uses. As yet production of reliably sterile and phenotypically uniform or predictable F1 hybrid seed for large-scale outplanting has not been achieved. Alternatively, sterile plants can be obtained from physical or chemical mutagenesis (Oladosu et al. 2016). Thus, exploration and evaluation of vegetative propagation are needed, especially for sterile mutations of leucaena with important agronomic attributes, such as high biomass.
There are published studies describing successful propagation of leucaena via rooted cuttings (Hu and Chih Cheng 1981; 8; Dick et al. 1998; Shi and Brewbaker 2006), grafting (Bray and Fulloon 1987; Brennan and Mudge 1998; ) and tissue culture or micropropagation (Dhawan and Bhojwani 1985; Puthur et al. 1998; Saafi and Borthakur 2002; Pal et al. 2012). Anecdotal reports indicate direct-planting of 2-m stakes may also be successful if harvested from saplings (Dahlanuddin pers. comm.; P. Larsen pers. comm.). Accessible guidebooks exist for setting up vegetative propagation systems for tropical woody plants that require relatively low sophistication or infrastructure (Longman 1993; Leakey 2012). Since each propagation method requires different levels of skill, labor, facilities and other resources and can generate different numbers of plants over time and space, there is a need for side-by-side comparisons to evaluate tradeoffs and make recommendations for specific contexts and end-uses. Addressing these needs was the objective of our study.
Materials and Methods
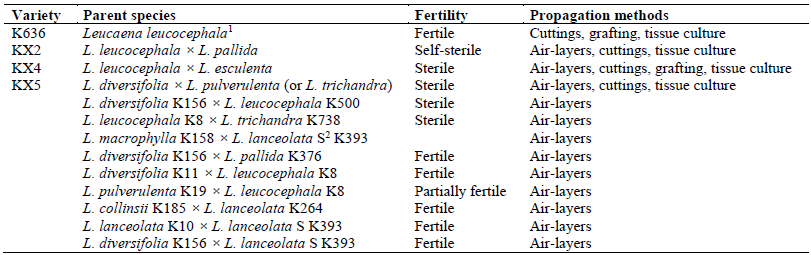
We compared several vegetative propagation methods, including air-layers (marcots), rooted cuttings, grafting and tissue culture (micropropagation) utilizing several self-sterile or fully sterile hybrids of leucaena and the cultivar K636 of L. leucocephala ssp. glabrata, as summarized in Table 1. Stock plants were field-grown clones of selected hybrids. Plants were pollarded to 1 m in height and allowed to resprout. New shoots were harvested for vegetative propagation when they were the required size for each method.
Seedlings
Seeds of L. leucocepaha ssp. glabrata, var. K636, were scarified in concentrated sulfuric acid for 15 min, rinsed in distilled water and germinated in 410 mL containers (‘D25l small deepots’, Stuewe & Sons, Tangent, OR, USA) filled with a commercial peat:perlite:vermiculite mixture (‘Pro-Mix HP’, Stuewe & Sons, Tangent, OR, USA). After germination, seedlings were placed in a secondary container and subjected to subirrigation twice daily to maintain adequate media water content. They were fertilized weekly using a liquid fertilizer mixture that was adjusted exponentially to match plant growth rates, as described in Idol and Diarra (2016).
Air-layering
We used ~3-month-old shoots to test air-layering. Vertical stems 4‒7 cm in diameter were girdled twice ~5 cm apart and the bark removed. Stems were girdled only during periods of adequate soil moisture to ensure sufficient sap flow and ease of bark removal. The cambium tissue near the upper girdle was treated with a commercial rooting hormone (Clonex gel, 0.31% indole-3-butyric acid, Growth Technology Ltd), wrapped in moist clean sphagnum moss, and covered with clear food-grade plastic wrap. Air-layers were checked for the presence of roots 3‒8 weeks after girdling. Girdled stems were vulnerable to breaking due to high or gusting winds.
Rooted stem cuttings
We followed the general protocol of Shi and Brewbaker (2006) to evaluate the success of rooted cuttings. Shoots were 3‒6-weeks-old when harvested. The first 2-node stem section with a fully formed leaf was harvested. We tested 2 rooting hormone concentrations and exposure times with variety KX4. The rooting hormone selected was a commercial liquid concentrate (Dip-N-Grow) that contains 1% indole-3-butyric acid (IBA) and 0.5% naphthalene acetic acid (NAA). The concentrations selected were 1,500 ppm (1,000 ppm IBA, 500 ppm NAA), 3,000 ppm (2,000 ppm IBA, 1,000 ppm NAA) and a control of distilled water. The freshly cut surface of the stem was then placed in the hormone solution for either 5 seconds or 5 minutes. Three rounds of cuttings were taken from the same clone of KX4 and the data aggregated to account for the effects of seasonality or repeatability on rooting success.
Once an optimal rooting hormone concentration and exposure time were determined, based on the first round of cuttings, we performed a second test to evaluate the effects on rooting success of position on the stem. Three consecutive 2-node cuttings were taken of variety KX4 and KX5, with the first cutting taken as described above. Three rounds of cuttings were taken to evaluate the repeatability of the results.
Finally, we tested the genetic variability of rooting success among 3 clones each of KX2 and KX5, both of which showed low rooting success in the study of Shi and Brewbaker (2006). The first 2-node cutting was used and subjected to the optimal rooting hormone concentration and exposure time. Three rounds of cuttings were taken to evaluate the repeatability of the results.
After exposure to rooting hormone, cuttings were placed in 107 mL conical containers (‘Ray Leach Conetainers’, Stuewe & Sons) filled with a 2:1 mixture by volume of vermiculite:peat moss. The cuttings were placed in a glasshouse with 50% shade cover and misted using aerosolizing mist nozzles (‘Arizona Mist’, Orbit Irrigation Products, North Salt Lake, UT, USA) set to deliver 15 seconds of misting spray every 5 minutes from 07:00 h to 19:00 h daily. Rooted plants were transplanted into 410 mL Deepots and treated as seedlings until they reached outplanting size.
Grafting
We attempted grafting of leucaena using 2 different-sized stems. For the first group, we used non-lignified shoots that were ~2 mm in diameter. For the second group, we used semi-woody shoots that were 4‒6 mm in diameter. In both cases, we used seedlings of variety K636 as the rootstock, and all leaves were removed from the scion. The diameter of the rootstock was matched as closely as possible to the diameter of the scion. For the first group (non-lignified shoots), we used a top-wedge graft to join the scion and rootstock and the stems were cut using a single-edge razor blade. For the second group (semi-woody shoots), we used 2 graft types. In the first round, scions of K636 and KX4 were grafted onto K636 seedling rootstocks using a top-wedge graft as was done with the smaller stems. In subsequent rounds, we grafted K636, KX2, KX4 and KX5 onto K636 rootstocks using a saddle graft created with a grafting tool that had a curved blade to create complementary cuts in the scion and rootstock. The graft union was wrapped in Parafilm and the grafted plant was covered with a polyethylene bag and placed in a controlled indoor environment with temperature ~25 oC and an artificial light source set to 12 h of simulated daylight (~240 μmol/m2/s of photosynthetically active radiation) in each 24 h period. Ten days later, the bags were removed and plants were moved into a mist environment as described for the rooted stem cuttings until new shoot growth on the scion was deemed vigorous enough that the graft union was successful and the plant could be transferred into the subirrigation system.
Tissue culture
We carried out a preliminary investigation of micropropagation to test the viability of vegetative material taken from field- and nursery-grown plants of sterile hybrids. Our starting material was excised stem sections containing an axillary bud from the first 6 nodes on green wood shoots that were 3‒4 weeks of age (i.e. the same age and size as those used for cuttings). Excised stem sections were surface-sterilized by placing them in a bleach solution for 10 min. Afterwards, they were rinsed in distilled water and placed on an agar medium in a sterile clear plastic container that was sealed and placed in a controlled environment with artificial lighting (12 h/d) and temperature ~25 oC. Plants were checked every few days for signs of microbial contamination, general health and the presence of new shoot or root growth.
Comparison of propagation techniques
Since KX4 was the most commonly used sterile hybrid for vegetative propagation, we selected it for comparison of the techniques according to several criteria, including:
the number of rooted plantlets that survived to out- planting size per field-grown stock plant;
the time taken from stumping or pollarding the stock plant until the plantlets reached outplanting size;
the approximate labor time required to generate plantlets and the labor skills required; and
the resources or facilities required to successfully carry out each method.
Data analysis
For rooted stem cuttings differences in rooting success of cuttings based on rooting hormone concentration, exposure time and stem position were analyzed using analysis of variance (ANOVA). Main effects were analyzed separately, i.e. we ignored the interaction effects. For stem position, only a single main effect was analyzed. Tukey's honest significant difference test was used to compare main effect means among treatment levels. For comparison of size between cuttings vs. grafted plantlets, we used a t-test assuming unequal variances.
Results and Discussion
Air-layers
Air-layers were highly successful (>90%) under conditions of adequate soil moisture and sap flow, but girdled stems were vulnerable to stem breakage owing to high wind gusts. Losses varied by stock plant and season but could be as high as one-third of stems over 6 weeks. Owing to their large size and vigorous growth after harvest, air-layers are suitable for direct planting in the field. They quickly outgrow containers if potted and used as stock plants in the nursery. Wind protection of air-layered stems is highly recommended.
We did not rigorously test direct outplanting of stakes in the ground, a common propagation practice with another widespread multipurpose legume tree, Gliricidia sepium (Simons and Stewart 1998). We know of only one published study, which is preliminary in nature (Duguma 1988); it reports resprouting of above-ground shoots but not direct evidence of rooting or long-term survival and growth. Anecdotal reports of success with stakes taken from saplings are promising, but it cannot be assumed stump sprouts from older trees will demonstrate similar success. We explored the viability of this technique with five 3‒6-month-old stump resprouts of seedless hybrids that are 20‒25 years of age. Harvested stems were planted 40 cm deep in moist soil, but none of the stems produced roots or persistent shoots after 3 months.
Rooted stem cuttings
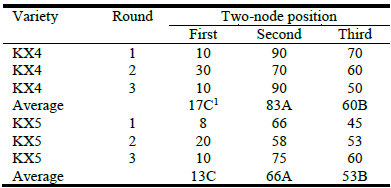
Rooting success of KX4 stem cuttings was highest at 1,500 ppm of rooting hormone concentration (Table 2) and there was no effect of exposure time. Success for control plants was <20%, averaged over 3 rounds of cuttings. The second stem position had the highest rooting success for both KX4 and KX5 (Table 3). Variety KX5 showed significant difference by clone in rooting success, but variety KX2 did not. Shi and Brewbaker (2006) had much lower success with these hybrids under standard propagation conditions. Only with the addition of an etiolation treatment of the stock plant did they achieve success rates >50%. As in their study, rooting success for cuttings taken and propagated during winter was much lower than in summer (data not shown).
Table 2 Rooting success (frequency, %) of KX4 stem cuttings at different rooting hormone concentrations and exposure times over 3 rounds of cuttings.

1Values for treatments followed by different upper-case letters and averages followed by different lower-case letters are significantly different (P<0.05).
Grafting
Grafting of the smaller stems (2‒3 mm diameter) was unsuccessful, while grafting of the larger stems (4‒6 mm diameter) with a top-wedge graft was more successful: 90% for K636 scion onto K636 rootstock and 60% for KX4 scion onto K636 rootstock. Grafting using a saddle graft created with the grafting tool was largely unsuccessful (<10%). While the tool provided rapid and complementary cuts on the scion and rootstock, the exposed tissue of the scion was not held tightly together by the rootstock as it was in a top-wedge or veneer graft. The blade on the grafting tool was also not as sharp as a razor blade or grafting knife, so it may have crushed some of the cambium cells as it sliced through the stem.
Tissue culture
Microbial contamination of growth media and excised nodes required an adjustment of the bleach solution and sterilization time to a final concentration of 50% bleach and 10 min exposure. In addition, the agar medium was supplemented with antibiotics (cefotaxime - 250 mg/L; carbenicillin - 100 mg/L; and rifampicin - 50 mg/L). Final contamination rates ranged from 15 to 25% for different hybrids. There were no differences by position along the stem. After 6 weeks, some plantlets initiated new shoot and root growth (Figure 1), demonstrating their viability for use in media supplemented with hormones to induce callous tissue and multiple stem formation as in Pal et al. (2012).

Figure 1 Examples of: (a) axillary bud stem sections used for micropropagation of sterile Leucaena hybrids; and (b) successful growth of new shoots and roots in sterile medium.
Table 4 lists the average number of successful plantlets per field-grown stock plant of KX4 for each technique and the average time required to produce a plantlet ready for outplanting. Rooted cuttings had the best combination of number of plantlets harvested per stock plant (2 cuttings per harvested stem), success rate and time to achieve outplanting size (16 weeks). Plant material was generally ready for harvest from the stock plant 3‒4 weeks after stumping or pollarding. The time to produce roots was approximately 4 weeks after placement in the mist system. After transplanting, rooted cuttings grew to outplant size in 8‒10 weeks. Ignoring stem breakage due to high winds, air-layers had the highest success rate but the fewest stems per stock plant that were appropriate for propagation. Stems used for air-layering were on average 12 weeks old, and root formation was usually vigorous within 4‒6 weeks. Stock plants produced about half as many suitable shoots for grafting as for rooted cuttings. However, this is based on a single point-in-time count, 4 weeks after pollarding. They also took longer to reach outplanting size (20 weeks).
Table 4 Number of plantlets per stock plant and time to outplanting for various vegetative propagation techniques. Seedlings of K636 included for comparison.
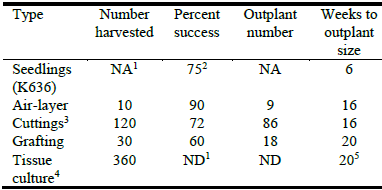
1NA = not applicable, ND = not determined; 2Starting from seed; 3Based on harvest of 4-week-old stems and use of 2 nodes per stem; 4Based on harvest of 4-week-old stems and use of 6 nodes per stem; 5Estimated using development and growth rates from Pal et al. (2012).
Assuming 6 usable nodes on 4-week-old stems, stock plants produced on average 360 suitable shoots for tissue culture. With a 15‒25% rate of contamination, that would result in ~250‒300 plantlets for multiple shoot and root induction. While we have not yet tested multiple shoot induction, if results from Pal et al. (2012) are used as a guide, we may expect ~200 rooted plantlets per stock plant that could be ready for outplanting within 20 weeks. Since micropropagation techniques can rely on repeated rounds of shoot induction from cultured plantlets, it requires only limited success from field- or nursery-grown stock plants initially to then scale-up production to whatever numbers are desired. The time to outplanting size would not be much different, since it takes only ~1 week for cut stems of field- or nursery-grown stock plants to break bud and produce new shoots.
The estimated labor requirements to propagate a batch of 100 plants to outplanting size varied from a low of 200 minutes for air-layers and cuttings to a high of 355 minutes for tissue culture (Table 5). The estimated time for 100 seedlings, by comparison, was 150 minutes. The distribution of labor among propagation activities also varied, based on the requirements of the methods. This also relates to the type of training and skills required, which would affect the total cost of production.
Table 5 Labor requirements (min/100 plantlets) for various vegetative propagation techniques. Seedlings of K636 included for comparison.
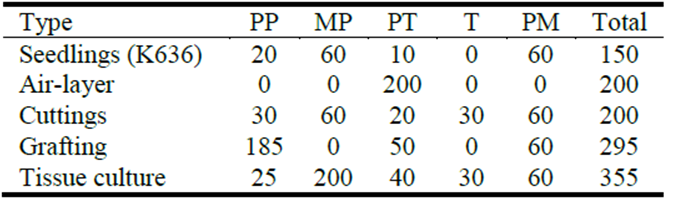
PP = Plant preparation; MP = Media preparation; PT = Propagation treatment; T = Transplanting; PM = Plant maintenance.
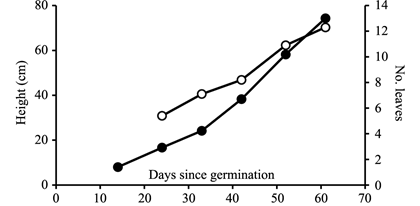
Grafted plantlets were significantly larger than rooted cuttings at outplanting for all measured variables and had a greater root:shoot ratio (Table 6). Seedling size at 10 weeks was larger than either grafts or cuttings at what we considered outplanting size. This was due to our desire to grow seedlings to a basal stem diameter of ~5 mm to use as the rootstock for grafting. Root mass and part of the shoot mass of grafts thus represent the contribution of the rootstock. Seedlings of K636 reached a comparable height as cuttings and grafts of KX4 in 6‒7 weeks after germination (Figure 2).
Table 6 Comparison of size at outplanting for rooted cuttings vs. grafted plantlets of sterile Leucaena hybrid KX4. Height and number of leaves for seedlings of K636 included as a standard. R:S, root:shoot ratio (‘shoot’ includes mass of leaves and stem).
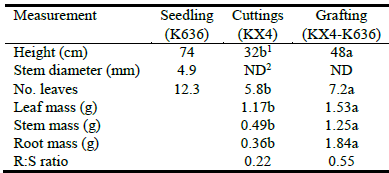
1Values followed by different letters are significantly different (P<0.05); 2ND = not determined.
Recommendations
Each technique has its relative advantages and disadvantages and thus can be recommended for specific purposes and contexts. Air-layering requires the fewest resources and no nursery phase for production. It also requires the least amount of labor to propagate and no maintenance of the stem from the time of propagation treatment to harvest. The rooted stems are also much larger and thus establish and grow faster after outplanting than other propagation techniques, including seedlings. The main drawback is the relatively low number of stems per stock plant (~10). Air-layering of other woody species is commonly done on stems 1‒2 cm in diameter, much smaller than we used in this study (4‒6 cm). We have experimented with air-layering of smaller stems of KX4 and even setting multiple air-layers on a single stem, but success rates are generally lower. We hypothesize that the upper limit for successful air-layered stems that can be propagated at ground level is 20‒30 per stock plant. If a producer starts with 20 trees as stock plants, then each round of propagation could produce 200‒600 air-layers, provided there is appropriate wind protection to minimize stem breakage. This is adequate for small-scale utilization, e.g. as field borders, wind breaks, cut-and-carry fodder for small animal production or as overhead shade for crops in a field 1 hectare or less in size. Thus, it can be recommended for on-farm propagation for smallholder producers.
If direct outplanting of resprouting stems ~2 m in length proves viable, it would avoid the problem of wind breakage with air-layers, but it would not significantly alter the number of stems per stock plant. It could reduce the time to outplanting from 16 weeks to 12 weeks, but vigorous growth of planted stakes would still be delayed by 4‒6 weeks as new roots would need time to form. Planting stakes deeper in the soil (50‒100 cm) than air-layers (~15 cm) increases the labor for outplanting, especially in clayey or rocky soils. However, it does avoid competition for water with surrounding herbaceous vegetation. Our preliminary results suggest low viability of this technique, although it may differ by variety or hybrid.
Grafting can be used for slightly greater production levels (30 per stock plant), if the producer or a propagator has an adequate outdoor or indoor nursery and a technician with skill and experience in simple grafting techniques. Compatible rootstock-scion combinations need to be established to ensure graft unions are successful and that stem growth of the rootstock and scion are reasonably similar. Given the possibility that air-layering could generate 20‒30 rooted stems per stock plant, grafting may seem unnecessary. However, the real advantage of grafting for leucaena is that a seedling rootstock can be used that has a healthy tap root to improve establishment, drought tolerance and recovery from regular browsing, pruning or other harvesting of the shoots. We have seen no decline in vigor of KX4 trees subject to pollarding 2‒3 times per year over an 8 year period. However, an integrated grazing or cut-and-carry fodder production system in Hawaii would ideally harvest shoot material 6‒8 times per year. The main disadvantage of grafting is the possibility of resprouting of the rootstock, which may represent either a suboptimal variety for forage production and quality or potentially a seeding variety that may be undesirable or restricted for outplanting in environ-mentally sensitive areas. The rootstock of Leucaena grafts will probably generate new shoots if the scion is browsed or pollarded, requiring attention from producers to prevent flowering and seeding of the rootstock. This is also a challenge in areas where seasonal frost may be significant enough to cause dieback of the stem below the graft union, i.e. close to the ground, as in subtropical areas of Australia or higher elevation sites in the tropics. Complete dieback of the scion would represent a failure of grafting as a propagation method.
Given the advantage of a tap root, the relatively low production level per stock plant is disappointing. Our early attempts to graft 2‒3-week-old stems of either KX4 or K636 onto the rootstock of K636 seedlings failed. However, Brennan and Mudge (1998) did have success with single-bud splice grafting of shoots 2‒3 mm diameter onto a similar-sized rootstock or via modified veneer graft onto rootstocks that were 5‒15 mm in diameter. If production of grafts could be increased to the range of rooted cuttings (100‒200 per stock plant), this could be a reasonable approach for commercial use beyond smallholder farms.
Rooted cuttings offer a balanced trade-off of rooted plantlets per stock plant, time to outplanting size and labor and skills requirements. The main drawback from our experience is the need for a mist system to protect the delicate leaflets from desiccation. Roger Leakey (2012, Ch. 7) describes and illustrates a standardized non-mist propagation chamber that can be constructed and used outdoors without the need for electricity or running water. It has been applied successfully for rooted cuttings of a number of tropical woody plants, but our attempts to replicate this in Hawaii with a variety of non-mist propagation chambers failed. We constructed a propagation chamber using Leakey's instructions and diagram, but even with overhead shade we were unable to keep the temperatures inside the chamber cool enough to sustain the cuttings and maintain high relative humidity (>90%). We tried 2 separate indoor non-mist propagation chambers with artificial lighting and controlled temperatures (25‒28 oC) and a chamber placed inside a glasshouse to provide natural lighting. The chambers maintained adequate humidity with once- or twice-daily hand misting, but cuttings still failed to thrive or produce roots. Thus, we settled on a mist system following guidance from Shi and Brewbaker (2006).
One intriguing alternative for rooted cuttings was reported by Dick et al. (1998). They harvested single-node cuttings sequentially down the stem of 1-yr-old seedlings of L. leucocephala, dipped the cuttings in a rooting hormone (0.8% IBA powder), and placed them in a non-mist propagation chamber with a heated bed to maintain temperatures of 22‒33 oC. They found very high success (near 100%) for nodes 5‒13 and success of >60% for 15 of 25 nodes evaluated. Stem diameter of nodes 5‒25 averaged 4‒7 mm, approximately the same as those we used for grafting. Our limited experimentation with stems of KX4 in this diameter range in a non-mist propagator was not successful. Seedling stems may retain a greater ability to produce roots from cuttings than stump sprouts, or there may be genetic differences between the hybrid KX4 and pure L. leucocephala that affect rooting success. This merits further study, since the ability to successfully propagate ~10 nodes per stem with a basal diameter of ~7 mm in a non-mist chamber could generate 200‒300 plantlets per stock plant, more than we achieved with leafy stem cuttings in a mist environment. The tradeoff is leafy stem cuttings can be harvested, beginning 3 weeks after stumping or pollarding, whereas stems 5‒7 mm in diameter with 15‒25 nodes require at least 6‒8 weeks of growth.
Assuming a combination of these 2 approaches could generate 200 rooted plantlets, this would produce ~4,000 plants from 20 field-grown stock plants every 6‒8 weeks. This would be sufficient for outplanting a hectare of pasture in an integrated grazing system, given a within-row spacing of 50 cm and an inter-row spacing of 5 m. Since our stock plants were on a 2 m spacing, a single hectare could generate 500,000 or more rooted cuttings in a single propagation round, enough for 120 hectares of pasture.
Our work with micropropagation of sterile leucaena hybrids is too preliminary to reliably predict success rates. However, the advantage of tissue culture is that once success is achieved in vitro, successive rounds of propagation can be performed without having to rely on stock plants in the field or nursery. Thousands of plantlets used for propagation can be kept in a sterile laboratory room with supplemental lighting. However, this means producers must rely on commercial sources for planting material, and propagators must have adequate facilities, reliable infrastructure, dependable supply chains and sufficient technical expertise. Where such conditions prevail, even difficult-to-root varieties may be eventually brought into production and propagated at whatever scale is desired. The other advantage is that in vitro samples can be easily shipped wherever there are adequate facilities for local propagation. For introducing or expanding integrated grazing systems in an area at a scale of hundreds of hectares per year, this would be the recommended method for vegetative propagation.
Conclusions
Leucaena has been successfully propagated vegetatively using most of the common techniques, including air-layering, rooted cuttings, grafting and tissue culture. Application to sterile hybrids of leucaena will be necessary for their widespread use until or unless there is commercial-scale availability of seed of reliably sterile F1 hybrid varieties. Based on our experience with several sterile hybrids, we can recommend air-layering for on-farm production of stock plants or for smallholder farmers interested in using leucaena as wind breaks, crop shade or cut-and-carry fodder for small animal production. Where adequate nursery facilities are available, rooted cuttings can be generated from plantations of stock plants at sufficient scale to supply larger farms, including extensive integrated grazing systems. Grafting provides plantlets the advantage of a seedling tap root and does not require indoor propagation facilities, but this reduces the number of scions per stock plant compared with cuttings. Tissue culture is yet to be successfully demonstrated with vegetative material from sterile hybrids, but our preliminary work suggests future success is likely. This would allow for mass propagation needed to establish integrated grazing at the scale of thousands of hectares per year and for easy sharing of material among cooperators for local production. Our comparisons among techniques in terms of production rates and times and required labor, skill and facilities provide a useful guide for selecting and refining methods appropriate for the scale of desired production as well as the end-use for this multipurpose tree.