Introduction
Leucaena leucocephala (leucaena) is a fast-growing tree-legume native to Southern Mexico and Central America (Brewbaker 1987). Leucaena is grown in the tropical and subtropical regions of the world for its multipurpose uses, which include fodder for farm animals and pulp for paper production. As a fodder, leucaena is highly palatable and rich in many micro- and macro-nutrients, including iron, fiber and protein (Brewbaker 2016). However, leucaena foliage can also contain high amounts of mimosine, a toxic non-protein amino acid, which is found in all plant parts, including foliage, flowers, seeds, stems, roots and root nodules (Soedarjo and Borthakur 1996). Mimosine toxicity is attributed to its ability to form a stable complex with pyridoxal-5’ phosphate (PLP) and metallic ions such as Fe3+, Cu2+ and Zn2+. These metallic ions and PLP are important enzyme co-factors for many biochemical pathways. Side-effects of mimosine toxicity resulting from disruption of these pathways include alopecia, infertility, fetal defects and goiter and other thyroid problems (Crounse et al. 1962; Hamilton et al. 1968; Joshi 1968; Dewreede and Wayman 1970). Mimosine is degraded to 3-hydroxy-4-pyridone (3H4P) by mimosine-degrading enzymes, mimosinase and rhizomimosinase, which are present in leucaena and Rhizobium sp. strain TAL1145, respectively (Negi et al 2013; 2014). Mimosine is also converted to 3H4P and 2,3-dihydroxypyridine (2,3-DHP) by the microflora present in ruminants (Dominguez-Bello and Stewart 1990). Both 3H4P and 2,3-DHP are also toxic but can be degraded to harmless products by the ruminal bacterium Synergistes jonesii (Jones and Megarrity 1986).
In spite of containing mimosine, leucaena is an ideal fodder due to the high protein concentration in its foliage, high fodder yield and resistance to many biotic and abiotic stresses, which include diseases, pests and drought (Shelton and Brewbaker 1994; Honda et al 2018). It is hypothesized that the changes in the mimosine concentration in leucaena are a response to environmental stresses. Therefore, considering its toxicity and possible role in stress resistance, it is important to study the fluctuation of mimosine concentrations in leucaena exposed to a range of environmental conditions.
Materials and Methods
Mimosine and 3-hydroxy-4-pyridone (3H4P) extraction and quantification
To extract mimosine and 3H4P from the various leucaena parts and tissues, 1 g samples of respective plant matter were placed in a 50 mL conical tube. The samples were then submerged in 30 mL of 0.1N HCl and incubated overnight at room temperature while shaking. Previous experience showed that heat and grinding treatments were unnecessary and less efficient for calculating the % dry weight of mimosine, so they were not used in the present study. After overnight incubation, leaflet extracts were spun for 15 min at 12,000 rpm to remove plant debris. The supernatants of leaflet extracts were assayed by HPLC using a Waters 2695 separations module, a Phenomenex C18 column (5μ; 4.6 × 250 mm), and a UV detection photodiode array (280 nm). An isocratic carrier solvent of 0.02 M o-phosphoric acid at a linear flow rate of 1 mL/min was used for HPLC analysis. The leaflet material was rinsed several times with dH2O then dried in a baking oven. For quantitative determination of mimosine and 3H4P, synthetic mimosine and 3H4P were prepared in various concentrations and then assayed by HPLC following the above methods. The areas under the curves for mimosine and 3H4P peaks were used to plot a standard curve, which was then used to quantify mimosine and 3H4P concentrations in leaflet extracts.
Mimosine in adult leucaena leaflets and shoot tips
Leucaena shoot tips, fresh leaflets and leaflets that had fallen from the plant and dried out, were collected, then separated based on color, size and health of the leaflets. Some fallen leaflets had a reddish color (due to loss of chlorophyll, oxidation and/or increase in pigments such as anthocyanins). Mimosine and 3H4P were extracted from the leaflets, then quantified by HPLC. In another experiment, fresh green leaflets were dried overnight in an oven before mimosine was extracted and quantified by HPLC. Experimental sets were performed in triplicate.
Mimosine in adult leucaena stems
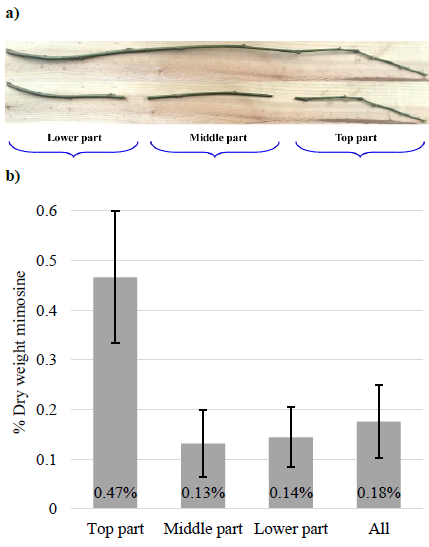
Stems that were no more than 6 mm in diameter from adult leucaena plants (common, i.e. shrubby variety) were harvested, then cut and separated into 3 parts, top, middle and lower sections. Mimosine was extracted from the various stem sections then quantified by HPLC. Each sample set contained 3 biological replicates.
Germination and growth of leucaena seedlings
Mature seeds of leucaena were collected from plants at the University of Hawaii Waimanalo Research Station, Waimanalo, Hawaii. Samples of seeds were submerged in concentrated sulfuric acid and gently agitated at room temperature for 6 min. After scarification, the seeds were rinsed with deionized water then placed in 51 × 25 cm trays containing a vermiculite-soil mixture. The seeds were allowed to germinate and then grew for 1 month at 25 ± 2 °C with a 16/8 h light/dark photoperiod with an irradiance of 74 μmol/s/m. Plants were watered once a week with quarter-strength Hoagland solution. After 1 month of growth seedlings were transferred to pots (4 per pot) containing a soil-vermiculite mixture. The seedlings were then grown for additional respective times following the methods described above. All treatments were carried out in these pots unless otherwise stated.
Treatment with NaCl
Quarter-strength Hoagland solution containing 300 mM NaCl was applied every 2 days to the growth media of 4-month-old leucaena seedlings. Mimosine was extracted and quantified from the leaflets of leucaena seedlings at 0, 3, 6, 9, 12, 15 and 18 days after initial application of NaCl treatment. Each sample set contained a minimum of 6 biological replicates.
Treatment with various metallic salts
Two-month-old leucaena seedlings were fed every 2 days with quarter-strength Hoagland solution containing water (control), 10 mM FeCl3, 10 mM ZnSO4 or 10 mM CaCl2. After 1 week of treatment, mimosine was extracted from leucaena leaflets, then quantified by HPLC. Each sample set contained a minimum of 4 biological replicates.
Treatment with various day lengths
Two-month-old leucaena seedlings were grown under hydroponic conditions for 5 days at 25 ± 2 °C under 16/8 h, 24/0 h or 0/24 h light/dark photoperiods. Mimosine was extracted and quantified from leucaena leaflets. In another experiment, 2-month-old leucaena seedlings were grown for 2 weeks at 25 ± 2 °C with a 16/8 h light/dark photoperiod under either white or purple light. Mimosine was extracted and quantified from the leaflets of treated leucaena seedlings. Each sample set contained at least 4 biological replicates.
Maceration of leucaena leaflets
One gram samples of mature leucaena leaflets were macerated in 20 mL of 0.1 N HCl at pH 1.8 (acidic solvent) or 0.1 M Tris-HCl at pH 8.0 (alkaline solvent) using a mortar and pestle. The macerated leucaena leaflets were incubated at room temperature and mimosine was quantified from the leaflet extract at 5, 10, 15 and 960 min after initial maceration. In another experiment, 1 g samples of mature leucaena leaflets were macerated in a mortar and pestle containing 20 mL of 0.1 M Tris-HCl buffered to pH 8.0 and containing water, 5 mM EDTA or 5 mM hydroxylamine. The macerated leaflet extracts were incubated overnight at room temperature and then mimosine and 3H4P were quantified in the leaflet extracts. The mimosine concentrations are shown as a percentage of fresh weight. Experimental sets were performed in triplicate.
Results
Mimosine and 3-hydroxy-4-pyridone (3H4P) concentrations in leucaena foliage
Among the various parts of leucaena foliage, mimosine concentration was highest in young shoot tips, which contained up to 22.2% mimosine on a dry weight (DW) basis followed by older shoot tips with 14.7% mimosine DW. Among the different leaflet sizes, younger and smaller leaflets contained a higher mimosine concentration than mature and larger leaflets (Figure 1).

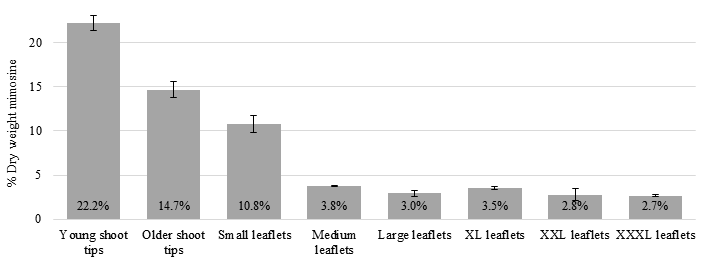
Figure 1 Mimosine concentrations in adult leucaena leaflets of various sizes and ages. Young shoot tips (shoot tip Y) contained higher concentrations of mimosine than older shoot tips (shoot tip O). Mimosine concentrations in leaflets appear to be correlated with leaflet size and age. Small and young leaflets contained significantly higher concentrations of mimosine than medium, large, extra-large (XL), extra-extra-large (XXL) and extra-extra-extra-large (X X X L) leaflets. Data are shown as a percentage of dry weight. Error bars indicate standard error from 3 replicates.
Mimosine and 3H4P concentrations in fallen red leucaena leaflets were 0.30% and 0.11% DW, respectively, which were lower than those of fallen green leaflets (Table 1, Figure 2). Fresh yellowish leaflets contained significantly less mimosine (1.4% DW) than fresh normal green leaflets (6.4% DW). Dried normal green leaflets had the lowest mimosine concentrations (0.1% DW) of all the leaflets tested. These results suggest that under certain conditions, a significant portion of the mimosine in leucaena leaflets is degraded to 3H4P.
Table 1 Mimosine and 3-hydroxy-4-pyridone concentrations in various types of mature leucaena leaflets.
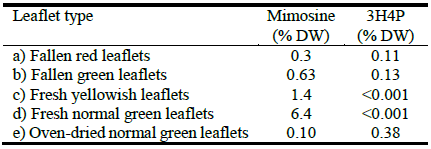

Figure 2 Mature leucaena leaflets: a) fallen red leaflets; b) fallen green leaflets; c) fresh yellowish leaflets; d) fresh normal green leaflets; and e) oven-dried normal green leaflets.
Concentrations of mimosine in the top sections of the stems were 0.47% mimosine (DW basis), which was significantly higher than for the middle and lower sections (Figure 3). The total mimosine concentration in the stems, including all sections, was 0.18% DW. These results indicate that the mimosine concentration is highest in the youngest and actively growing parts of the stem.
Mimosine concentrations in leucaena seedlings grown in NaCl solution
The mimosine concentrations in leucaeana leaflets extracted at 0 and 3 days of NaCl treatment remained relatively low at 2.44 and 2.37% DW, respectively. However, at 6 days of treatment, the mimosine concentrations had increased significantly (3.25% DW) and remained relatively high throughout the rest of the treatment time (Figure 4). This suggests that mimosine synthesis and accumulation may increase under saline conditions, possibly as a stress response.
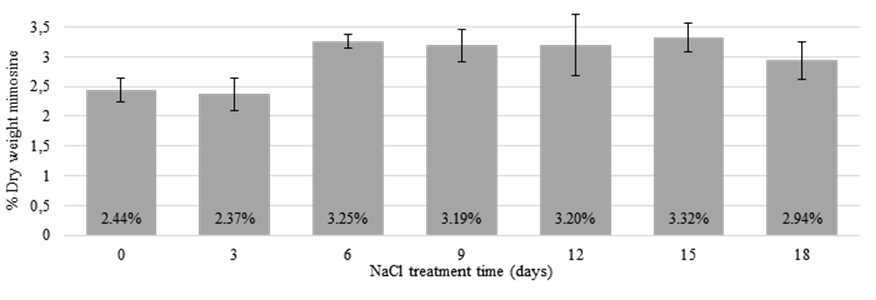
Figure 4 Mimosine concentrations in leucaena leaflets treated for 18 days with 300 mM NaCl. At 0 and 3 days of NaCl treatment, the mimosine concentration remained relatively low; however, from 6 to 18 days of treatment, the mimosine concentration had increased and remained relatively high. Data are shown as a percentage of dry weight. Error bars indicate standard error from minimum of 6 biological replicates.
Mimosine concentrations in leucaena seedlings after growing for 1 week in the presence of metallic salts
Treatment of leucaena seedlings with 10 mM FeCl3 or 10 mM ZnSO4 had no significant effects on the mimosine concentrations in leucaena leaflets when compared with the untreated controls (Figure 5). However, leaflets of leucaena seedlings treated with CaCl2 had a lower mimosine concentration than the untreated controls. These results indicate that synthesis and accumulation of mimosine may not be a stress response following exposure to metallic ions like Fe3+ and Zn2+. Degradation of mimosine or inhibition of the synthesis of mimosine may be a response by leucaena to exposure to excessive Ca2+.
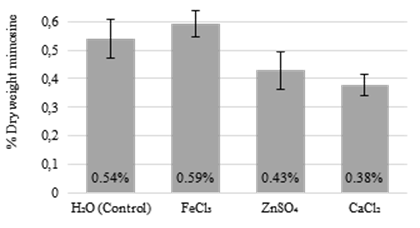
Figure 5 Mimosine concentrations in leucaena leaflets treated for 1 week with 10 mM metallic salts. Treatment with FeCl3 and ZnSO4 did not significantly change the mimosine concentrations in leucaena leaflets relative to the control. However, treatment of seedlings with CaCl2 lowered mimosine concentration relative to the control. Data are shown as a percentage of dry weight. Error bars indicate standard error from minimum 4 biological replicates.
Mimosine concentrations in leucaena seedlings after exposure to various light treatments
Interestingly, 2-month-old seedlings exposed to 5 days of complete darkness and 5 days of total light had higher mimosine concentrations than the control plants, which were exposed to normal light/dark photoperiods (16/8 h, light/dark; Figure 6a). This suggests that excessive light or dark may stimulate mimosine synthesis and accumulation in leucaena. In the other experiment, 3-month-old leucaena seedlings grown under white light had higher mimosine concentrations (2.71% DM) than plants grown under purple light (1.97% DM) (Figure 6b). These results indicate that duration of light exposure and light color can affect the mimosine concentrations in leucaena plants.
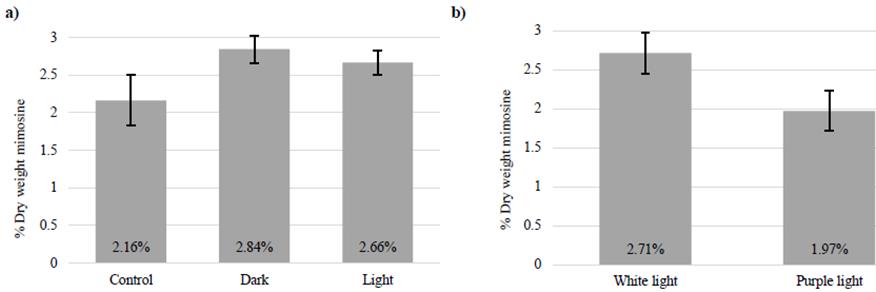
Figure 6 a) Mimosine concentrations in leucaena seedlings grown for 5 days with 16/8 h (control), 0/24 h (dark) or 24/0 h (light) light/dark photoperiods; and b) Mimosine concentrations in leucaena leaflets from seedlings grown for 2 weeks with 16/8 h light/dark photoperiods under white or purple light. Leucaena leaflets contained a higher mimosine concentration when grown under white light versus purple light. Data are shown as a percentage of dry weight. Error bars indicate standard error from minimum 4 biological replicates.
Mimosine concentrations in leucaena leaflets after maceration
Figure 7a shows that when leucaena leaflets were macerated and incubated in solvent buffered to pH 1.8, mimosine concentrations remained high. However, when leaflets were macerated and incubated in solvent buffered to pH 8.0, mimosine concentrations decreased significantly over time. These results indicate that maceration of leaflets induces degradation of mimosine, possibly due to the release of the mimosinase enzyme from the chloroplast. Figure 7b shows that maceration solvents containing hydroxylamine contained significantly more mimosine than solvents containing water or EDTA. These results indicate that the mimosinase enzyme is inhibited by hydroxylamine but not by EDTA.
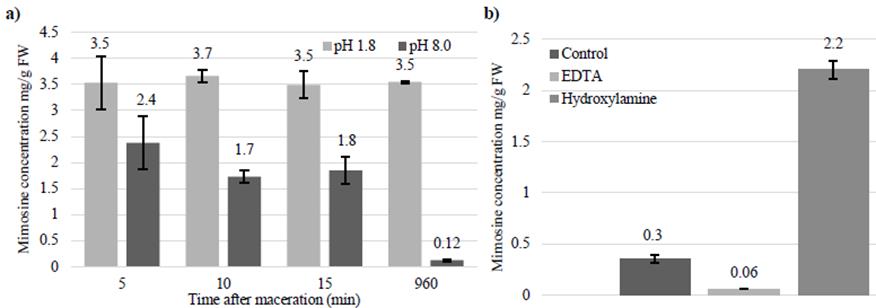
Figure 7 a) Mimosine concentrations in leucaena leaflet extracts after maceration in solvents buffered to pH 1.8 or pH 8.0 and incubated for 5, 10, 15 and 960 min. The mimosine concentrations of leaflet extracts decreased when it was macerated in solvent buffered to pH 8.0, but did not change when macerated in solvent buffered to pH 1.8; and b) Mimosine concentrations in leucaena leaflet extracts after maceration in solvent buffered to pH 8.0 and then incubated overnight. The mimosine concentrations remained high in solvents containing hydroxylamine, but not in solvents containing water or EDTA. Data are shown as a proportion of fresh weight (mg/g). Error bars indicate standard error from 3 replicates.
Discussion
Mimosine concentration in leucaena leaflets appears to be correlated with the size and age of the tissue. Young shoot tips, small leaflets and growing stems contained a higher mimosine concentration than larger leaflets and older portions of the stem. Mimosine and its degradation product, 3H4P, are known to have antimicrobial, nematicidal and insecticidal properties (Anitha et al. 2005; Nguyen et al. 2015; Xuan et al. 2016). A high mimosine concentration in young and actively growing portions of leucaena may be an evolutionary adaptation to protect it from browsers, and pest and pathogen attack. Herbicidal properties of mimosine have been studied and it has been shown to inhibit germination of rice, wheat and sicklepod seeds (Prasad and Subhashini 1994; Xuan et al. 2006; Williams and Hoagland 2007). Defoliation of leucaena leaflets, which contain both mimosine and 3H4P, may be a strategy to release these compounds into the soil as a means of inhibiting pathogens and preventing the growth of potential plant competitors.
The increase in leaf mimosine concentrations in leucaena seedlings grown in media treated with 300 mM NaCl relative to the untreated controls at 6‒18 days of treatment may be an adaptation to salt or osmotic stress. NaCl can change the osmotic pressure of plant roots and induce drought-like conditions. In order to prevent water loss due to drought stress, plants accumulate neutral solute compounds called osmolytes (Nahar et al. 2016), which include carbohydrates, polyhydric alcohols, methylamines and free amino acids like valine, proline, isoleucine and aspartic acid (Burg and Ferraris 2008). Mimosine may serve as an osmolyte to prevent water loss, when leucaena is under osmotic stress.
Surprisingly, the mimosine concentrations in leucaena seedlings grown for 5 days in complete darkness (0/24 h light/dark photoperiod) were higher than those in control plants (16/8 h light/dark photoperiod). Darkness as an extreme light condition can induce leaf senescence, which can lead to a decrease in proteins, photosynthetic activity and chlorophyll (Fujiki et al. 2005; Song et al. 2014). Soudry et al. (2005) found that both detached and attached Arabidopsis leaves had increased amino acid concentrations during senescence and suggested that the increased free amino acids may be a result of proteolysis. The increased mimosine concentrations in leucaena seedlings exposed to darkness may be the result of leaf senescence. Although mimosine is a non-protein amino acid, it is synthesized from O-acetylserine (OAS) and 3H4P in a reaction catalyzed by mimosine/cysteine synthase (Yafuso et al. 2014). During leaf senescence, proteolysis may be induced, possibly resulting in increased free serine levels, which could cause a rise in OAS levels, leading to an increase in the mimosine concentration. Increasing the mimosine concentration during prolonged darkness may be a strategy by leucaena to accumulate additional metabolites that can be utilized at a later time.
Leaflets that have fallen from plants and dried out, and oven-dried leucaena leaflets both contained significantly less mimosine than fresh leaflets. Under these conditions, mimosine is likely to be degraded by mimosinase, resulting in a lower mimosine concentration. Similarly, maceration of leucaena leaflets in an alkaline-buffered solvent caused a decrease in the mimosine concentration. This decrease is also possibly due to mimosine degradation by mimosinase after being released from the chloroplast upon maceration. Mimosinase is a PLP-dependent carbon-nitrogen lyase that degrades mimosine into 3H4P, pyruvate and ammonia (Negi et al. 2014). Mimosinase has high enzyme activity at pH 8.0 and very low activity below pH 6.0 (Negi and Borthakur 2016). This would explain why maceration of leucaena leaflets in solvent buffered to pH 8.0 resulted in significantly lower mimosine concentrations than in leaflets macerated in solvent buffered to pH 1.8. This study shows that either drying leucaena leaflets or macerating them in an alkaline solution can significantly reduce the mimosine concentration in leucaena foliage. For farmers concerned about mimosine toxicity, utilizing one of these methods may help to lower the mimosine concentration in leucaena foliage, which could also lead to an increase in the nutrient profile of leucaena used for fodder. Honda and Borthakur (2019) identified a number of genes that were highly expressed in the foliage of leucaena compared with the roots and postulated that these genes may contribute to the nutrient richness of leucaena foliage.
The mimosine concentration in leucaena is affected by environmental conditions, indicating that mimosine synthesis, degradation and accumulation fluctuate with environmental conditions. Negi et al. (2014) postulated that mimosine serves as a carbon and nitrogen reserve, which is accumulated during conditions of high nutrient availability, and is degraded during periods of low nutrient availability, such as during drought. As previously mentioned, mimosine may serve leucaena as an osmolyte to help it retain moisture under osmotic stress. Rodrigues-Corrêa et al. (2019) found that mimosine accumulates in giant leucaena in response to various stress elicitors. In the same study, they found that mimosine had the ability to quench free radicals and limit oxidative damage in foliar discs of bean plants. Osmotic and oxidative stresses are secondary stresses induced by primary biotic and abiotic stresses (Wang et al. 2003). The changes in the mimosine concentrations in leucaena may be a response mechanism to help it cope with the secondary stresses induced as a result of a primary stress. One possible explanation for the ability of leucaena to tolerate a wide range of environmental conditions might be that it produces large amounts of mimosine, which may serve multiple roles in stress tolerance.