Introduction
Teff (Eragrostis tef), also known as lovegrass or annual bunch grass, is an ancient gluten-free cereal crop mostly adapted to environments with warm seasons (Spaenij-Dekking et al. 2005; Assefa et al. 2015). Teff originated in Ethiopia (Ketema 1993; Girma et al. 2014), and has been introduced to regions and countries with a range of climates, including the USA, India, Africa, Western Europe and Australia (Ketema 1997; Girma et al. 2014) owing to its importance for supplying good quality proteins for human use and for animal feeding as a forage or silage crop (Hagos and Melaku 2009; Zhu 2018).
Teff seeds are the world’s smallest cereal grain, with an average l,000-seed weight of 0.3‒0.4 g (Kreitschitz et al. 2009). The market value of Teff seed is determined mainly by seed color, which varies from white to dark brown (Ketema 1993). An often temporary state of physiological seed dormancy means many cereal seeds either do not germinate or have low germination percentage (Khan et al. 2017), which prevents a successful start to the season and uniform stand establishment in several important plant species including Lolium rigidum (Steadman et al. 2003), Elymus elymoides (Meyer et al. 2000) and Bromus tectorum (Bauer et al. 1998). Some research into Teff seed has been conducted including hydrothermal time modelling (van Delden 2011), plus grain structure and properties of caryopses of Teff seeds (Kreitschitz et al. 2009). However, there is still a lack of information about the severity of innate seed dormancy, and the effects of stress-related plant hormones and light on germination performance of Teff seed.
Seed priming, or osmoconditioning, which is influenced by several factors such as temperature, water potential and duration, is a relatively common method for treating seed to initiate seed germination. Priming is done prior to the emergence of the primary root during later seed imbibition (Pill 1995), thereby enhancing germination under conditions marginal for establishment. Osmotica used in priming include polyethylene glycol (PEG), sodium or potassium salts (e.g. K3PO4, KH2PO4, MgSO4, NaCl), which control water uptake of seeds during seed imbibition and can provide a higher germination percentage and rate than unprimed vegetable and grass seeds (Frett and Pill 1995). Previous reports indicated that dormancy in freshly harvested seeds of Amaranthus cruentus (Tiryaki et al. 2005), Poa pratensis (Tiryaki et al. 2006) and Phacelia tanacetifolia (Tiryaki and Keles 2012) could be eliminated, to some extent, by priming in the presence of stress-related plant hormones like methyl jasmonate (JA-Me), 1-amino-cyclopropane-1-carboxylic acid (ACC) and 6-benzyl-amino-purine (BAP) (Tiryaki and Buyukcingil 2009).
The objectives of this study were to determine the presence and severity of innate seed dormancy of Eragrostis tef seeds, and to reveal the effects of stress-related plant hormones on germination performance of the seeds when incubated with and without light.
Materials and Methods
Material
Eragrostis tef (accession PI 197210) seeds used in the experiments were harvested by hand from plants grown under field conditions on 12 October 2017, in Canakkale, Turkey. Harvested seeds came from a single lot and were immediately used for germination experiments at Canakkale Onsekiz Mart University, Canakkale.
Methods
Pre-experimental trials indicated the best priming medium and duration of priming were 1% KNO3 for 24 hours (Tiryaki unpublished data). A single layer of seeds (0.3 g) was placed on double layers of filter paper and 3 mL of 1% KNO3 was applied plus various concentrations of the following primers: acetyl salicylic acid (ASA ‒ 1, 5, 10 and 15 μM), methyl jasmonate (JA-Me ‒ 0.3, 0.6, 0.9 and 1.2 μM), gibberellic acid (GA3 ‒ 50, 100, 150 and 200 μM) and indole-3-acetic acid (IAA ‒ 0.5, 1.0, 1.5 and 2.0 μM) for 24 h at 21 ( 0.5 ºC in darkness. Prior to the germination test, the priming solution was removed from the primed seeds by washing with tap water for 1 minute and then placing seeds on paper towels for 2 h under room conditions. Surface-dried and primed seeds were then used for the germination experiments. Seeds treated with 1% KNO3 only, seeds treated with distilled water (dH2O) only (hydropriming) and non-primed seeds were used as controls.
Germination experiments were conducted in a growth chamber held at 21 ± 0.5 °C either in constant light (210 μmol/m2/s for 24 h/d) or darkness. A completely randomized block design with 4 replications of 50 seeds was arranged as a factorial. Replicates of each treatment were placed either in different shelves of the same growth chamber or were arranged in different positions in light-sealed boxes in light and dark experiments, respectively, and were randomized after each count. Seeds, which showed a radicle exceeding 2 mm in length emerged from the testa, were counted as germinated and were removed daily from the petri dishes for 7 days in light and 12 days in dark germination experiments. The final germination percentage (FGP) was determined and its angular transformation (arcsine√FGP) was used to provide a normal distribution for data analysis. Germination rate (estimated as days to 50% of FGP) and the span of germination (time from 10% to 90% of FGP) were calculated from the total number of seeds germinated using a method used successfully on sugarbeet seeds (Murray et al. 1993).
SAS statistical software was used to analyze the data (SAS 1997) and Fisher’s least significant difference (LSD) test was applied, if the F test was significant at P<0.05.
Results
Effects of priming media and plant hormones in darkness
Approximately half of the Teff seeds were considered dormant shortly after harvesting when incubated in the absence of light. Priming seeds in 1% KNO3 for 24 h at 21 ( 0.5 ºC in the dark significantly improved germination percentage over that of untreated control seeds (89.4 vs. 56.5%, respectively; Figure 1). The results also revealed that hydropriming significantly reduced FGP and gave the lowest FGP (40.5%) in comparison with untreated control seeds.
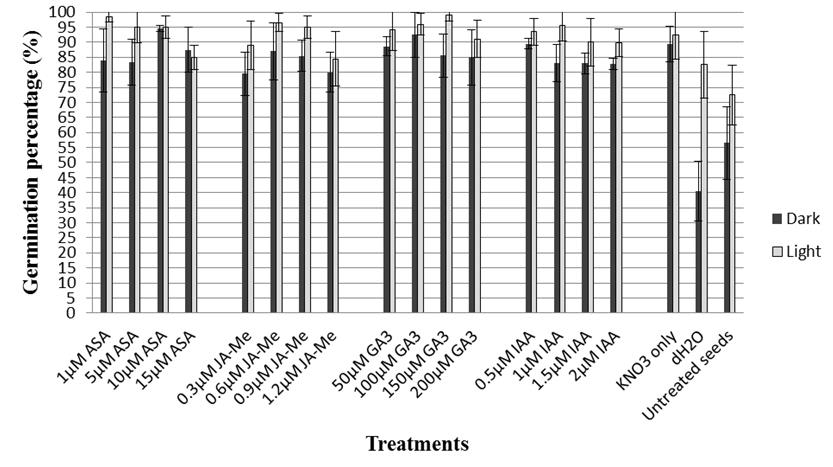
Figure 1 The final germination percentage (FGP) of Teff seeds primed in 1% KNO3 supplemented with various concentrations of different plant hormones for 24 h at 21 °C and germinated under light or dark conditions at 21 °C. Bar indicates SD (n = 4).
The inclusion of plant hormones in the priming medium generally had little effect on FGP with values ranging from 79.5 to 94.5% (Figure 1).
Priming in the presence of hormones generally had little effect on the speed of germination of seeds, while seeds primed in dH2O had the slowest (G50 = 3.76 days; P<0.05) seed germination (Table 1).
Table 1 The angular transformation [FGP] of final germination percentage (FGP), speed (G50) and span (G10-90) of Teff seed germination incubated in dark or light at 21 °C following priming for 24 hours at 21 °C in 1% KNO3 combined with different plant hormones at various concentrations.
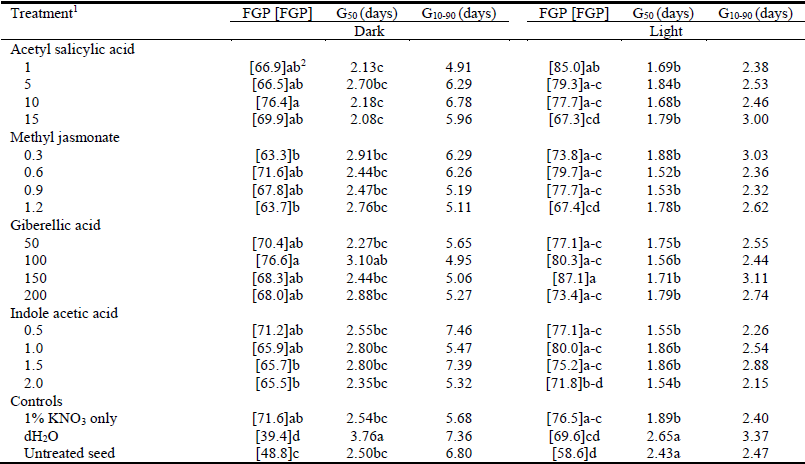
1KNO3 + related plant hormone at given concentrations (μM).
2Values within columns followed by different letters differ (P<0.05).
Effects of priming media and plant hormones in light
In general, seeds germinated in light had higher FGP than seeds germinated in the dark (Figure 1). In light, priming seeds in 1% KNO3 significantly improved FGP compared with untreated control seeds (92.5 vs. 72.5%; Figure 1). Adding 150 (M GA3 and 1 (M ASA to the priming medium (Figure 1) improved FGP to 99 and 98.5%, respectively, while adding the other plant hormones provided intermediate results. Hydroprimed seeds had significantly higher FGP (82.5%) than untreated control seeds (72.5%), which was in complete contrast to the results obtained in darkness (Figure 1).
Seeds primed in 1% KNO3 had significantly enhanced germination rate (G50 = 1.89 days) compared with untreated control seeds (G50 = 2.43 days) (Table 1), while there was no further improvement from inclusion of plant hormones (Table 1). Hydroprimed (G50 = 2.65 days) and untreated (G50 = 2.43 days) control seeds had the slowest germination speeds (Table 1). There were no significant differences between primed and control seeds for span of germination (G10-90) in either light or dark germination conditions (Table 1).
Discussion
One of the most important prerequisites for a successful plant stand is even, fast and successful seed germination. This can depend on external factors such as light, temperature and water supply to the seed as well as endogenous seed factors including ripeness (seed maturity), hormone levels and other forms of dormancy (van Delden 2011). Of those, seed dormancy can delay and spread seed germination over time, and is a characteristic of several plant species (Schonbeck and Egley 1981; Gu et al. 2003). Several methods are used to reduce or overcome seed dormancy, and these can vary among species (Steadman et al. 2003; ). Priming seeds in an appropriate osmoticum improves germination and seedling emergence of several plant species (Tiryaki and Keles 2012), and a positive photoblastism, light-inducible seed germination, was recently reported for Eragrostis ciliaris (Khan et al. 2017). Although existence of slight embryo dormancy was previously mentioned in fresh seeds of Eragrostis abyssinica [synonym of E. tef (Zuccagni) Trotter] (Katayama and Nakagama 1972; van Delden 2011), this study has demonstrated that E. tef has light-inducible seed germination and half of freshly harvested seed can be dormant. In addition, the study revealed that untreated control seeds had higher FGP (72.5%) in the presence of light than untreated control seeds germinated in darkness (56.5%). Priming of dormant seeds in 1% KNO3 for 24 h at 21 ( 0.5 ºC significantly improved FGP and provided faster seed germination in the presence of light (Figure 1), while the same priming treatment had no effect on the span of germination in both light and dark conditions (Table 1). In addition, hydropriming appeared to decrease FGP (40.5%) in darkness in comparison with untreated control seeds (56.5%), whereas hydroprimed seeds had a higher FGP (82.5%) than untreated control seeds (72.5%) in the light (Figure 1). These results suggest that hydropriming of Teff seed could be considered if planting alone or in a mixture with other grasses as a seeding material for temporary nurse grass or erosion control.
Light is one of the most important regulatory factors of seed germination for many plant species including reed canary grass (Lindig-Cisneros and Zedler 2001), weedy rice (Lee et al. 2010), Arabidopsis (Auge et al. 2018), lettuce (Sawada et al. 2008), tomato (Auge et al. 2009) and phacelia (Tiryaki and Keles 2012). Positively photoblastic species often have small and dormant seeds (Floresa et al. 2011). Previous reports indicated that seed dormancy was influenced by the nitrate concentrations in both soil and seed (Matakiadis et al. 2009; Huang et al. 2018) and phytochrome plays a very significant role in this action (Footitt et al. 2013). However, this may not be the case in Teff seeds since seeds primed in KNO3 gave about the same FGPs with (92.5%) and without (89.4%) light during incubation (Figure 1). It may be that use of KNO3 as a priming agent with Teff seeds enhances seed germination by balancing endogenous nitrate content or by accelerating the decrease in abscisic acid (ABA) level prior to the completion of germination triggered by the production of N-related signals (Footitt et al. 2013).
It is well known that the level of physiological seed dormancy is influenced by ABA and GA (giberellic acid) homeostasis during seed maturation (Seo et al. 2009). Although the exact dormancy-release mechanisms of the hormones are still unknown for many plant species (Nakabayashi et al. 2012), recent new molecular approaches partially shed light on these mechanisms (Shen et al. 2018; Auge et al. 2018). In general, inclusion of plant hormones with priming solution used in this study did not provide any further improvement on FGP under both light and no light conditions except for ASA and GA3 at certain concentrations. A high concentration of ASA (10 (M), which had FGP of 94.5%, appeared to be more effective under no light conditions, while the lower concentration of ASA (1 (M) provided a better improvement in FGP under light conditions (98.5%) (Figure 1). In contrast, a higher GA3 concentration (150 (M) gave a higher FGP (99.0%) in the light than the seeds primed in the presence of 100 (M GA3 (92.5%) under no light conditions. The differences are relatively small, but indicate germination and dormancy-release processes of Teff seeds may be influenced by ASA and GA homeostasis with light playing an important role in this regulation. A similar regulation of ASA and GA was previously shown for lettuce (Sawada et al. 2008) and pepper seeds (Korkmaz 2005).
Recent advances in genetic and biochemical studies have revealed that GA metabolism of seeds is controlled by light-response (Varbanova et al. 2007; Footitt et al. 2017). Involvement of light and GA in seed germination was also previously shown in Arabidopsis at the molecular level (Ogawa et al. 2003). Those authors suggested that increased levels of GA in germinated seeds were controlled mainly by both transcriptional activation and repression of GA biosynthetic as well as GA catabolic genes (Ogawa et al. 2003). However, this is not the case for Teff seeds, since exogenous application of ASA and GA3 produced similar additional increments in FGP under both light and no light conditions (Table 1).
Jasmonic acid (JA) or methylated jasmonic acid (JA-Me) affects several biological activities in plants, including plant growth and development, as well as responses to biotic and abiotic stresses (Tiryaki and Staswick 2002). Previous reports indicated that exogenous applications of JA or JA-Me stimulate germination of dormant seeds in various plant species (Berestetzky et al. 1991; Ranjan and Lewak 1992), while non-dormant seed germination was inhibited in some other plant species (Corbineau et al. 1988; Daletskaya and Sembdner 1989).
In this study we found that JA-Me neither stimulated nor inhibited the germination percentage of dormant E. tef seeds when primed with KNO3. This may indicate that JA-Me has no, or only a limited, function in the germination processes of Teff seed. It is well known that JA-Me and IAA have signaling cross talk to control or to regulate several important plant growth parameters, including response to light (Westfall et al. 2016; Kućko et al. 2017; Sherp et al. 2018). Regulatory involvement of these two plant hormones in seed germination was previously shown in wheat (Xu et al. 2016), pepper (Korkmaz 2005), Kentucky bluegrass (Tiryaki et al. 2006) and Arabidopsis (Shu et al. 2016). Altogether the results of this study suggested that JA-Me and IAA neither regulate Teff seed germination nor are involved in N-related signals with ABA (Footitt et al. 2013; Jacobsen et al. 2013), further physiological or molecular studies are needed to confirm these results.
In conclusion, this study indicates that Teff has light-inducible seed germination and a substantial proportion of freshly harvested E. tef seeds can be dormant. This can be overcome to some extent by priming seeds in 1% KNO3. The results also revealed that including ASA and GA3 in priming media may play a role in enhancing the Teff germination process. These findings have possible implications for treatment of seed following harvesting and prior to sowing.















