INTRODUCTION
Reynolds syndrome (RS) is an autoimmune disease characterized by overlapping primary biliary cirrhosis (PBC) and limited cutaneous systemic sclerosis (lcSSc); it affects women mainly, and shows an incomplete form of scleroderma. Usually, PBC, in the context of RS, is associated with symptoms that evolve slowly and that are tolerated by patients; it also implies a good prognosis if compared with scleroderma or PBC alone, although diagnosis may be delayed.
While the prevalence of pulmonary arterial hypertension (PAH) in systemic sclerosis patients is about 12% -with a significant related mortality rate at 3 years after the diagnosis of PAH 1- the prevalence in some studies increases to 26% in RS, negatively affecting prognosis 2. Some published cases do not report PAH, and diagnosis is based on clinical, immunological and histological findings, focusing mainly on dermatologic features 39, associated Sjogren's syndrome 10 or as an uncommon presentation of a malignant thymoma 11.
Only one case of reported PAH was found, but it was an image report 12. The case presented here not only some of the features mentioned above, but also severe PAH as part of a study on dyspnea in a woman with both lcSsc and PBC, who was frequently misdi-agnosed with an exacerbation of chronic obstructive pulmonary disease.
CASE PRESENTATION
75-year-old woman from Bogotá (Colombia), hospitalized in 2014 due to a history of one month of dyspnea, aggravated by 2 weeks of orthopnea, and purulent expectoration. Further research found that the patient pre sented with generalized pruritus, erythema, non-foveal edema in lower limbs and pyrosis the year before. The patient's history showed exposure to tobacco smoke (12.5 pack-year) and alcohol intake once a week since her twenties, as well as chronic bronchitis managed with oxygen (the patient stopped using the oxygen and did not have a prior spirometric test) that required hospitalization with adequate recovery. The patient did not have a diagnosis of diabetes mellitus, systemic arterial hypertension, nephropathy or hepatopathy. No family history was disclosed.
Clinical findings
Since 2009, the patient had persistent cough without expectoration for more than three months, and dyspnea class III according to the functional classification by the New York Heart Association (NYHA), which eventually progressed to NYHA IV, with orthopnea and paroxysmal nocturnal dyspnea; additionally, she experienced productive cough with purulent expectoration for 2 weeks before consultation.
Further investigation revealed that the patient had a previous history of Raynaud's phenomenon and pyrosis. Initial cardiorespiratory examination showed a heart rate of 66 beats per minute, arterial pressure of 108/58 mmHg (systolic/diastolic pressure), respiratory rate of 19 breaths per minute, perioral cyanosis, third degree jugular venous distention, loud tricuspid S2 sound, respiratory distress that required using accessory muscles, coarse crackles in both lungs, hepatomegaly and non-foveal edema in the lower limbs painful on palpation.
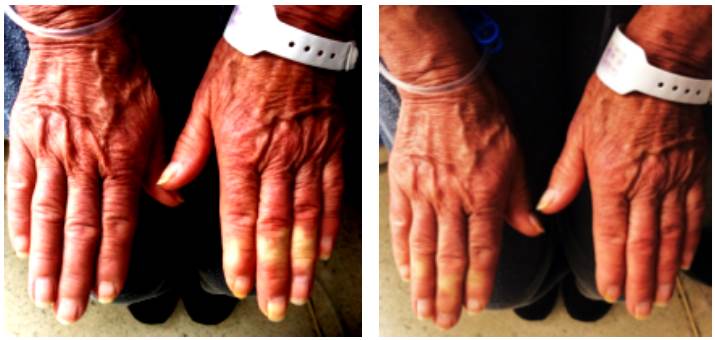
Physical examination confirmed secondary Raynaud phenomenon, and generalized pruritus, which was her main symptom, affecting the thorax, abdomen, back and legs. Skin inspection showed nasal telangiectasias, pruritic erythema in abdomen, back and limbs with signs of scratching, red hands, and sclerodactyly (Figure 1).
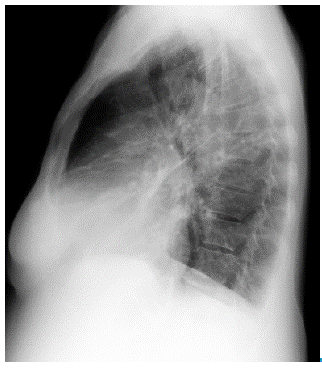
Initial studies included total blood count (normal), electrolytes (sodium:137 mEq/Lt, potassium: 3.82 mEq/Lt, chlorine:103 mEq/ Lt), urine test (normal), arterial blood gases interpreted at 560mmHg atmospheric pressure, which showed a chronic compensated respiratory acidosis without oxygenation disorder (pH 7.38; partial pressure of carbon dioxide (pCO2): 38.9mmHg; bicarbonate (HCO3): 23.6 mE-q/L; base excess: -1.7; oxygen partial pressure (pO2): 95.1mmHg; ratio PaO2/FiO2: 297, lactate 0.8 mmol/L), creatinine and blood ureic nitrogen (1.1 mg/dl and 29.25 mg/dl, respectively). Chest radiography showed an increased cardiothoracic index and signs of pre-capillary pulmonary hypertension (Figure 2a and 2b).

Source: Own elaboration based on the data obtained in the study.
Fig 2A RChest radiograph. Posteroanterior view.
Source: Own elaboration based on the data obtained in the study.
Fig 2B Left lateral view of chest radiograph. Both projections show pneumonia in the anterobasal segment of the left lower lobe and inferior lingular segment, right middle lobe atelectasis, enlarged cardiac size with pleural effusion and pericardial effusion. Additionally, signs of precapillary pulmonary hypertension can be observed.
Liver profile studies were conducted (Table 1) showing slightly high bilirubin (conjugated: 0.38 mg/dL (47%); unconjugated bilirubin: 0.42 (53%) mg/dL; total: 0.8 mg/dl) and transaminases (ALT 80.5 U/L, AST 82.6 U/L) with high levels of alkaline phosphatase (357 IU/L, reference value: < 250 IU/L); partial thromboplastin time: 34.5 sec (control 32.6), prothrombin time: 16.7sec (control 12.9), and serum albumin test: 3.21 gr/dL.
Table 1 Hepatic and immunological tests.
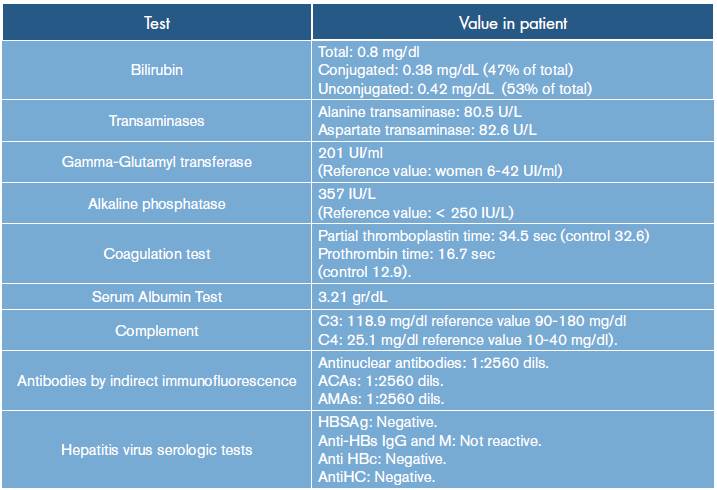
ACAs: anti-centromere antibodies; AMAs: antimitochondrial antibodies; HBSAg: hepatitis B surface antigen; Anti-HBs: hepatitis B surface antibody; Anti HBc: total hepatitis B core antibody; Anti HC: hepatitis C antibody.
Source: Own elaboration based on the data obtained in the study.
An incomplete cholestasis was suspected, and a complete ultrasound of the abdomen was requested, revealing congestive hepatomegaly, free fluid in the peritoneal cavity, and thickening of the gallbladder wall. Measuring GGT levels in blood was determined to confirm intrahepatic cholestatic injury, obtaining a high result (201 UI/ml; reference value: women 6-42 UI/ml).
Initially, decompensated heart failure by an infectious pulmonary process was considered due to the symptoms and the findings obtained during physical examination, which were associated with a chronic pulmonary pathology. The acute infection was treated with antibiotic therapy (piperacillintazobactam for seven days) and low-flow oxygen therapy, obtaining some improvement of dyspnea, purulent expectoration cough and absence of crackles on pulmonary auscultation.
A chronic pulmonary pathology was considered as a probably diffuse interstitial process -based on the findings related to nasal telangiectasias, sclerodactyly, Raynaud phenomenon and on the radiographic evidence of pulmonary hypertension (classified as type I)- due to limited systemic scleroderma (or CREST syndrome), which along with incomplete cholestasis, elevated transaminases and pruritus, increases the possibility of an overlapping primary cirrhosis.
A chest radiograph showed increased cardiothoracic index, sign of pre-capillary pulmonary hypertension, which was confirmed by high-resolution computed tomography (HRCT); no pulmonary fibrosis was found. Also, HRTC was compatible with pneumonia in the antero-basal segment of the lower left lobe, with pleural and pericardial effusion. A sputum smear was performed searching for M. tuberculosis, but it yielded negative results.
Cardiovascular studies included an electrocardiogram (Figure 3) with a first-degree atrioventricular block and right bundle branch block without signs of hypertrophy, ischemia or infarction. Transthoracic echocardiography revealed a depressed left ventricular systolic function (ejection fraction of 45%) and relaxation disorder, also confirming cor pulmonale with severe pulmonary hypertension (sPAP=73 mmHg) and tricuspid regurgitation grade IV/IV. By epidemiological nexus with Chagas disease, a Chaga test was requested, but it reported a negative result.
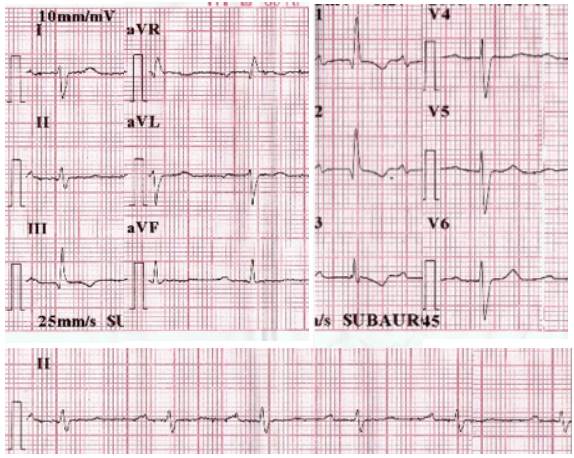
Source: Own elaboration based on the data obtained in the study.
Fig 3 12-lead electrocardiogram. Findings include first-degree AV block and right bundle branch block.
In addition, an upper gastrointestinal endoscopy test was performed, reporting erythematous mucosa in the esophagus and antral gastritis.
The diagnosis considered limited systemic scleroderma, with the possibility of primary biliary cirrhosis overlap as the cause of intra-hepatic cholestasis; immunological tests were performed to confirm a common autoimmune disorder. Also, serum complement levels were measured, showing normal values, which helped ruling out other conditions characterized by complement consumption (C3: 118.9 mg/ dl. Reference value: 90-180 mg/dl; C4: 25.1 mg/dl. Reference value 10-40 mg/dl).
Antinuclear antibodies by indirect immunofluorescence showed positive results [antinuclear antibodies: positive with anti-centromere antibodies (ACAs) = 1:2560 dilutions. Anti-mitochondrial antibodies (AMAs) = 1:2560 dilutions)], confirming the presence of two specific patterns of antinuclear antibodies (ANA) for localized scleroderma and primary biliary cirrhosis; viral hepatitis serologic tests were negative.
Based on these findings, the patient was successfully treated with an angiotensin receptor blocker (Losartan), proton pump inhibitors (meprazole) and antihistamines (Loratadine) to minimize the effects of the pathology; also, Sildenafil -a selective phosphodiesterase-5 inhibitor- was administered at 50 mg twice a day for 4 weeks to treat type I PAH, which improved respiratory symptoms. After being discharged, the patient was referred for outpatient monitoring by internal medicine and rheumatology, preserving the treatment and, subsequently, achieving the improvement of symptoms.
DISCUSSION
This patient presented with cor pulmonale secondary to a type 1 pulmonary arterial hypertension, with scleroderma associated to primary biliary cirrhosis or Reynolds syndrome. Systemic sclerosis is a heterogeneous systemic connective tissue disease characterized by vascular endothelial damage in small vessels, autoimmune response associated with specific auto-antibodies, and progressive fibroblast dysfunction leading to an increased deposition of the extracellular matrix. The main clinical manifestations include skin thickening and involvement of internal organs (e.g., gastrointestinal tract, lungs, heart, kidney, central nervous system).
According to the ACR-EULAR criteria for the classification of systemic sclerosis, patients with a total score of nine or more are classified as definite systemic sclerosis; skin thickening in the fingers of both hands with extension to the proximal metacarpophalangeal joints completes the criteria for diagnosis 13.
Moreover, the liver is a lymphoid organ involved in the immune response and in the maintenance of tolerance to self-molecules, but it is also a target for autoimmune reactions, as observed in primary biliary cirrhosis (PBC) 14. PBC is a chronic cholestatic disease, of unknown cause, which shows a slow and progressive destruction of small intrahepatic bile ducts, impaired biliary secretion and stasis of bile acids within the liver that can produce liver fibrosis and cirrhosis 15. This disease causes the increase of serum alkaline phosphatase - more than 1.5 times above the normal limit- and is characterized by the presence of anti-mitochondrial antibodies (AMAs), liver histology with non-suppurative destructive cholangitis, and destruction of interlobular bile ducts. A patient who meets two of these three criteria is diagnosed with PBC 16.
Mid and late 50s is the average age of onset in various studies, and women are affected by this condition more frequently 17. Furthermore, PBC can be associated with many immunological disorders like systemic lupus erythematosus (2.7-15%), Sjogren syndrome (35-57%), serum anti-phospholipid antibodies (75%) and systemic sclerosis (the prevalence of systemic sclerosis among patients with PBC is 7-12%, while PBC has been reported in 2.5% of SSc cases 14). In the case presented in this paper, the patient had previously shown dermatologic and respiratory symptoms related to scleroderma, and laboratory findings were associated with the mitochondrial pattern of antinuclear antibodies 18; increased phosphatase alkaline without a high level of bilirubin suggests an asymptomatic or an early stage of autoimmune liver pathology 19-21, therefore, PBC was overlapping SSc.
Reynolds syndrome is a rare autoimmune disease with a possible laminopathy genetic substrate 22, and consists in the simultaneous presence of progressive systemic sclerosis and primary biliary cirrhosis. It was first described by Dr. Reynolds in 1970, who reported six female patients with pruritus, jaundice and hepatomegaly with marked elevation of serum alkaline phosphatase activity, and a positive test for serum mitochondrial antibody 23. Lamins are ubiquitous proteins that polymerize to form nuclear lamina, a meshwork of intermediate filaments located under the inner nuclear membrane; they are involved in laminopathies, a heterogeneous group of diseases that share clinical similarities with SSc. A single heterozygous missense mutation in the Lamin B receptor, LBR exon 9 (c.1114C/T; p.R372C), leads to a change from a hydrophilic amino acid (arginine) to a hydrophobic amino acid (cysteine) 24.
According to Stadie 25, the coincidence of progressive systemic sclerosis and primary biliary cirrhosis seems to be a favorable association for the progression of primary biliary cirrhosis; in this case, the good prognosis of RS could be affected by a pulmonary complication 26.
PAH is another aspect included in the ACR-EULAR criteria for the classification of systemic sclerosis. It is defined as an elevated mean pulmonary artery pressure (mPAP) -25 mmHg- with a pulmonary capillary wedge pressure of 15 mm/Hg. The prevalence of PAH among patients with SSc varies among different studies but rounds 10% 27. In a meta-analysis including twelve studies, the pooled prevalence estimate of PAH in patients with systemic sclerosis was 13 % (95% CI, 8.96% to 17.87%) with an 12 figure of 95.5 % (95% CI, 94.1% to 96.4%), using a random-effects model for estimation.
The estimated prevalence of PAH reported in patients with connective tissue diseases was of 13 % (95% CI, 9.18% to 18.16%) ranging between 2.8 % and 32 % 28. In this case, respiratory symptoms were the main complaint of the patient, and pulmonary hypertension was documented in the study because the patient did not tolerate withdrawal of oxygen (since she had clinical signs of pulmonary hypertension, echocardiography was requested evidencing hypertension). Moreover, tomography did not show interstitial lung disease as a cause of PAH.
In consequence, looking for type 1 PAH in a patient with RS is important because this association is related to poor outcomes. Observational studies have demonstrated that mortality remains high in SSc patients with PAH; more specifically, the three-year survival rate for SSc patients with PAH has been estimated in 56%, compared with 94% in those without PAH, therefore, constituting itself as a leading cause of morbidity and mortality 1) (26,29,30.
A systematic review suggests that using transthoracic echocardiogram (TTE), pulmonary function tests, and NT-ProBNP for screening and diagnosis of SSc-PAH 31, in the presence of a low lung diffusing capacity for carbon monoxide (DLCO) (45-70%), is associated with a 5.6-7.4% of PAH development; on the other hand, a decline in DLCO is associated with an increase in the specificity for PAH (DLCO < 50%, specificity = 90%).
In the DETECT study 29, where a population with SSc was studied, nomograms for practical application of the algorithm were proposed to determine the likelihood of pulmonary arterial hypertension and to decide whether to take a TTE, and the subsequent right heart catheterization, with a sensitivity of 97%. This study included seven non-echocardiographic variables (predicted FVC%, predicted DLCO%, current/past telangiectasias, serum anticentromere antibody, serum N-terminal probrain natriuretic peptide (NTproBNP), serum urate, and right axis deviation on ECG) with a prediction model for PAH of 84% [area under the receiver operating characteristic curve (ROC AUC) 95% CI: 79.5 to 89.8] 29. In this case, only predicted FVC%, predicted DLCO%, and NTproBNP were not included.
According to ESC/ERS guidelines for pulmonary hypertension 30 "the therapy of PAH patients cannot be considered as a mere prescription of drugs; it is characterized by an interdisciplinary strategy and the combination of different drugs plus interventions" 31; thus, physical activity, nutritional and vaccination recommendations, along with the referral to a rheumatologist and pneumologist, were given to the patient. In addition, a pharmacologic therapy was initiated.
The treatment of PAH begins with the treatment of the underlying cause when possible, but in type 1 PAH, options include endothelin receptor antagonists (Bosetan or Ambrisetan) or phosphodiesterase type-5 (PDE-5) inhibitors 32. PDE-5 inhibitors are involved in this process through the inactivation of cyclic guanosine monophosphate, the second messenger of the nitric oxide pathway 33-35; it is expressed in lung tissue, and may be upregulated in PAH.
Sildenafil citrate is a selective PDE-5 inhibitor, which is orally active and potent according to a clinical trial (SUPER-1) 36 and its extended study (SUPER-2) 37. PAH patients treated with Sildenafil 20, 40, or 80 mg t.i.d. have reported good results on 6-min walk distance, improving and maintaining functional class 38, haemodynamics parameters 37, and renal function (increased glomerular filtration rate, decreased serum creatinine) 38.
Finally, this study reports the following limitations: 1) the right heart catheterization (RHC) was not performed despite the high level of sPAP in the patient, considering that the error estimation of the right echocardiography is ±20mmhg 39; 2) nailfold capillaroscopy was not available in the institution but the 'SSc pattern' was documented, including architectural disorganization, giant capillaries, haemorrhages, loss of capillaries, angiogenesis and avascular areas, which characterize >95% of patients with overt scleroderma 40; 3) magnetic resonance cholangiography of the liver and hepatic biopsy were not performed because they were not available in our second level medical center. In spite of these limitations, this case significantly contributes to knowledge on SR with some unusual clinical features.
In conclusion, dyspnea could be caused by many conditions, but in the presence of clinical and physical findings that suggest an autoimmune disorder, scleroderma should be considered, and PAH should also be investigated since it is present in up to 10% of patients, conferring a worse prognosis if confirmed. Additionally, an internist should keep in mind that, after appropriate clinical review and physical examination, not only the most frequent diagnoses should be considered but also differential diagnoses bearing in mind autoimmune diseases, especially when there are various organs affected. "Low prevalence" diseases can be overlooked.