INTRODUCTION
Paracoccidioidomycosis (PCM), also known as South American blastomycosis, was first described in 1908 by Adolfo Lutz. This is a chronic granulomatous disease caused by the dimorphic fungus known as Paracoccidioides brasiliensis. It is endemic in Latin America, with predominance in Brazil -with the highest incidence in the southeast of the country- followed by Venezuela, Colombia, Ecuador and Argentina. 1. The dimorphic fungus grows as a yeast in the tissues of the host and in cultures at 36-37°C, but it develops as a slow growing mold at temperatures <28°C 2.
This disease compromises mainly the lungs, but can spread to other organs, with particular trophism, through oral mucosa, adrenal glands, reticuloendothelial system, skin and bones. 3 This paper reports a case of pulmonary PCM in an immunocompetent patient -a rare disease in Colombia- who was diagnosed in a tertiary care hospital in Santander, Colombia, and had a fatal outcome.
CASE PRESENTATION
67-year-old mestizo male from the rural area of the municipality of Aratoca (where temperatures vary between 16°C and 26°C), who worked as a farmer (mostly in coffee crops), with unclear pathological history of epilepsy without treatment, chronic smoking associated with regular consumption of alcoholic drinks, and without exposure to individuals with a history of tuberculosis.
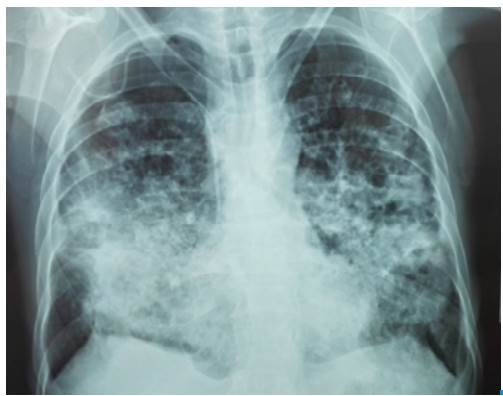
The patient visited a primary health center referring symptoms of 4 months evolution including productive cough with mucopurulent expectoration, progressive dyspnea even when putting small efforts, fever, chills and unintentional progressive weight loss. He stated that the symptoms exacerbated 7 days before with hemoptoic cough and evening diaphoresis. The patient was referred to a secondary care health center where a chest x-ray was performed, showing abundant alveolar opacities in both pulmonary fields, formation of diffuse pneumatoceles, and signs of air trapping (Figure 1). A sputum KOH test was performed, reporting a double refractory wall of yeast with intracytoplasmic vacuoles with multiple or chain budding. The results were compatible with paracoccidioides, so he was assessed by the internal medicine service and referred to the Hospital Universitario de Santander due to the high risk of ventilatory failure.
Source: Own elaboration based on the data obtained in the study.
Figure 1 Chest x-ray with abundant alveolar opacities in both lung fields with formation of pneumatoceles.
On physical examination at admission, the patient presented with respiratory distress at rest despite supplementary oxygen with a mask and Ventury mask with a 50% inspired fraction, significant reduction in muscle mass, vital signs with normal blood pressure, a heart rate of 106 bpm and respiratory rate of 23 breaths per minute. He did not have a feverish state at the time of assessment, but there were intercostal retractions with diminished respiratory sounds on auscultation and rhonchi with diffuse rales.
High-resolution computed tomography (HRCT) of the chest was performed (Figure 2), which showed multiple cavitations, mostly thin-walled, and randomly distributed nodules with a predominantly right pleural effusion.
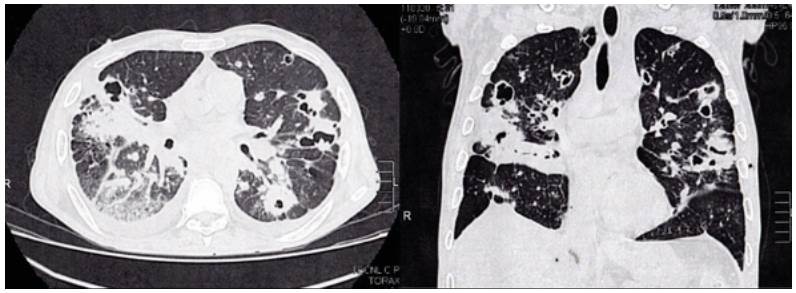
Source: Own elaboration based on the data obtained in the study.
Figure 2 High-resolution computed tomography of the chest.
The initial tests ruled out infection by human immunodeficiency virus through fourth-generation ELISA technique. Three serial sputum smears were performed, which reported negative results for acid-alcohol resistant bacillus (BAAR). In addition, a polymerase chain reaction (GenXpert) was performed for each bacilloscopy sample, yielding negative results for BAAR as well. A sputum KOH test was performed again, which confirmed the presence of blastoconidia related to P. brasiliensis.
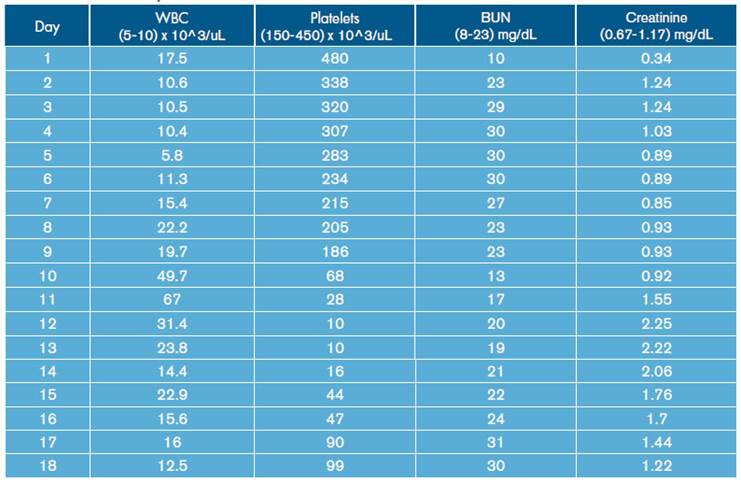
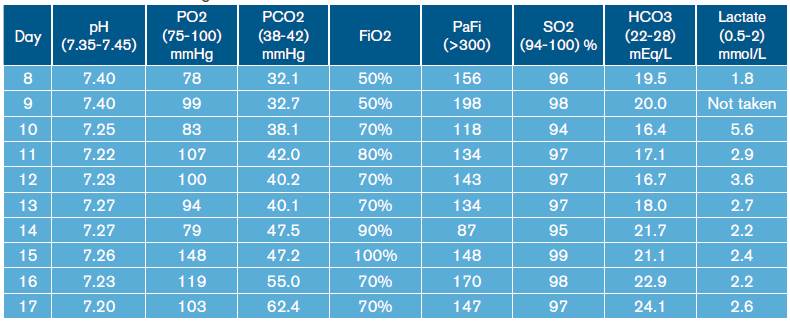
Similarly, leukocytosis, progressive thrombocytopenia and deterioration of renal function were documented (Table 1). Blood gas tests were made, showing hypoxemia with hypercapnia associated with mixed acid-base disorders due to normochloremic metabolic acidosis, metabolic alkalosis and respiratory alkalosis (Table 2).
During hospital stay, the patient was assessed by pneumology, indicating treatment with amphotericin B deoxycholate at a dose of 0.7-0.8 mg/kg/day. On day 12 of hospitalization, the patient presented increased respiratory function and worsening of hypoxemia (Table 2), invasive mechanical ventilator support requirement, hypotension refractory to fluid management, and vasopressor support with norepinephrine was initiated. In addition, control laboratories reported significant leukocytosis and thrombocytopenia without bleeding (Table 1). Bronchial secretion and blood cultures reported growth of Klebsiella pneumoniae with antimicrobial resistance pattern of extended-spectrum beta-lactamase (ESBL), for which antibiotic treatment with Meropenem was prescribed.
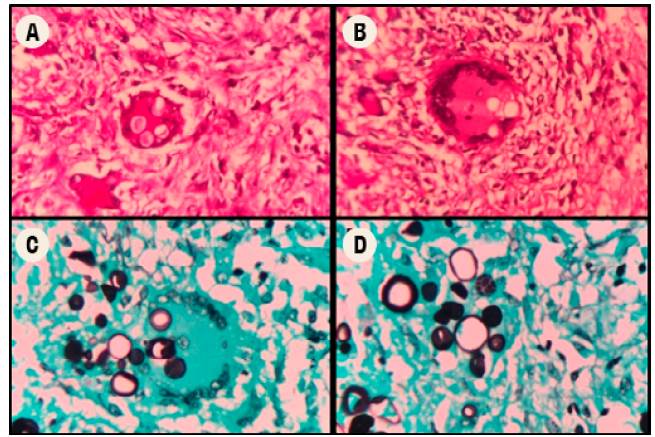
The subject was taken to a medical-scientific autopsy, in which histopathological studies were carried out, including cuts of the lung parenchyma (Figure 3), showing the presence of granulomas with abundant multinucleated Langhans giant cells and refractory rounded structures in the cytoplasm. Some of the latter had multiple budding and were compatible with P. brasiliensis through periodic acid-Schiff (PAS) staining and methenamine silver stain (GMS). This finding was also observed in lymphatic and hematopoietic system cuts, while no foci of pneumonia caused by K. pneumoniae were identified. The renal parenchyma showed diffuse and generalized necrosis of the epithelium of the proximal tubules, secondary to tissue hypoperfusion and triggered by septic shock.
DISCUSSION
This paper reported a case of PCM in a patient with undefined structural lung disease, with subsequent complication due to septic shock associated with K. pneumoniae. This disease is found in Latin America, with 80% of the cases reported in Brazil, followed by Colombia, particularly Santander and Norte de Santander, which are considered endemic areas. The national incidence ranges from 0.1 to 2.4 per million inhabitants and mortality is approximately 10-15% 4-7.
P. brasiliensis is a dimorphic fungus 2 that affects mostly individuals who are engaged in agricultural activities because of the manipulation of soils that generate aerosols with spores of this fungus, which end up being inhaled by the farmers. In addition, a greater incidence is observed in the male gender, with a male-to-female ratio of 2:1 3. This difference may be caused by the fact that men are more dedicated to agricultural activities and the protective effect of estrogen in women, thus avoiding the transition of the fungus from mycelium to yeast 2. The transmission from person to person of this mycosis has not been documented, and tobacco and alcohol consumption has been associated with the risk of PCM infection 8.
PCM is a chronic systemic invasive mycosis that, like tuberculosis, mainly affects the lungs; both diseases can coexist in 1520% of cases 9,10 with varying degrees of parenchymal injury and tendency to fibrosis 2. Given this possible coexistence, tuberculosis was one of the first pathologies to be discarded in this patient, besides an immunodeficiency state as a trigger of the infection.
Moreover, the association with other pulmonary structural processes, such as chronic obstructive pulmonary disease and lung cancer, has been described 11. In this case, the association of PCM, in the absence of tuberculosis, and the bacteremia by K. pneumoniae was striking. Although the concurrent process of tuberculosis and pulmonary PCM 11 and pulmonary tuberculosis and bacterial pneumonia have been documented 12, the association of pulmonary PCM and K. pneumoniae infections is not frequent. In a series of reference cases, only one presented the concomitant process 13. Although this patient did not comply with the description of the CD40L phenotype and HIV infection was ruled out, the presence of structural lung disease should be highlighted, as well as his malnutrition status, smoking and active alcohol consumption, hospital stay -which favors nosocomial infections-, and his exposure to drugs with potent adverse effects such as amphotericin B -which favors a possible poor immune response.
The presence of non-modifiable risk factors such as age and sex, together with modifiable factors such as type of job, smoking and consumption of alcoholic drinks, associated with a low socioeconomic level and high degree of malnutrition conditioned a depressed immune response and subsequent infection by P. brasiliensis11. However, mycosis per se modulates the host's innate and acquired immune response, so that overlapping or co-existing infections may be facilitated.
Chest scans are the main tool to suspect this infection. Findings are nonspecific and include diffuse micronodular infiltrates predominantly in the middle zone of the lung, cavitations and tumor masses 5,14,15. Chest radiography shows mainly interstitial opacities (nodular or reticular), and when caverns are observed, pulmonary tuberculosis is the main differential diagnosis. 15,16. In high-resolution chest tomography, untreated PCM findings are characterized by attenuation of the ground-glass lung parenchyma associated with small centrilobular nodules, cavity nodules, large nodules, and scar emphysema 5,15,16, being the peripheral and the posterior distributions in the lung predominant 16. The reversed halo sign is observed in about 10% of cases 16.
Considering the wide differential diagnosis 17 and the clinical-radiological dissociation 18, a rapid confirmation of the diagnosis is required to initiate treatment. These tools include microbiological, immunological and molecular evaluation methods 19. The reference diagnostics are:
Pulmonary tuberculosis
Histoplasmosis
Systemic lupus erythematosus
Hodgkin lymphoma
Cutaneous and mucosal Leishmaniasis
Wegener's granulomatosis
Actinomycosis
Blastomycosis
Microscopic evaluation of sputum or compromised tissues allows rapid diagnosis. The use of routine stains, such as potassium hydroxide (KOH), calcofluor for wet mounts and Grocott-Gomori staining or periodic acid-Schiff (PAS) for smear 20, allows to visualize spherical or double-walled yeasts, from 30μ to 60μ in diameter, with multiple budding 14 . Digested or concentrated sputum can be positive in 60-70% of chronic PCM cases 15. Cultivation of the fungus on Sabouraud dextrose agar medium is ideal for isolation, but may take 20 to 90 days 14,15,21. In certain cases, microbiological documentation of the presence of PCM is not possible, so, in case of clinical and radiological suspicion, the use of serological methods is necessary.
The detection of antibodies against the gp43 antigen is carried out by means of an immunodiffusion reaction, which is positive in 90% of the patients prior to the eradication treatment 19,22,23. In HIV patients, results should be interpreted with caution due to cross-reaction with Histoplasma capsulatum24. Another option is to detect the p27 antigen, with a sensitivity and specificity of almost 100% 5,20, which avoids cross reaction 20. Molecular methods based on the polymerase chain reaction technique are not commercially feasible yet 20.
The treatment of P. brasiliensis differs from other invasive fungi due to its high sensitivity to different antifungal medications; the selection should be made according to the severity of the disease (Table 3).
Table 3 Treatment options for pulmonary paracoccidioidomycosis.
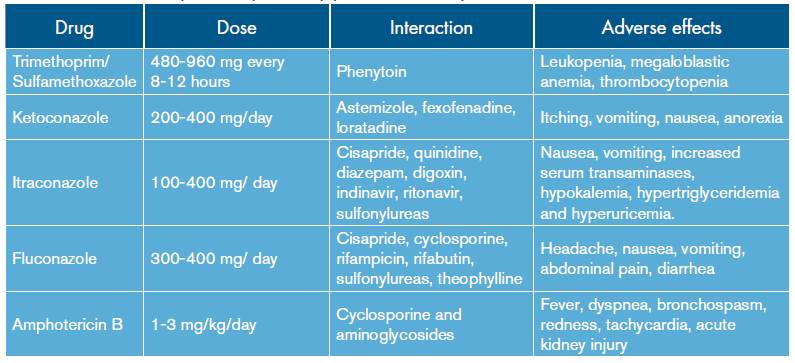
Source: Own elaboration based on data obtained in the study.
In cases of mild to moderate infection, the therapeutic option is itraconazole. The literature reports a 90% cure rate with relapses up to 15%. One treatment option is to combine trimethoprim and sulfamethoxazole 25. In case of serious infections, the use of intravenous agents is indicated, being amphotericin B in its conventional or lipid formulation the preferred choice 14,15,19 -as administered to this patient- evaluated at a dose of 1-3 mg/ kg/day. Similarly, in order to prevent relapse, long-term treatments with sulphonamides or azoles are indicated 15.
Although PCM is an endemic entity with high incidence and mortality, few studies have been carried out to define the appropriate therapeutic option 15. In addition, both the adverse effects and the interaction of medications that may affect its effectiveness must be considered 10.
CONCLUSIONS
Pulmonary paracoccidioidomycosis is an endemic disease in Santander, although it is underdiagnosed due to the presence of more common pathologies and similar clinical characteristics such as tuberculosis and histoplasmosis. For this reason, late diagnosis is frequent in most of the affected individuals. Unfortunately, mycosis modulates the immune, innate and acquired response of the host, thus facilitating the onset of superimposed or co-existing infections, whose complications such as sepsis and secondary multiple organ failure eventually lead to a fatal outcome.
The timely diagnosis of PCM is of utmost importance, considering that the ideal diagnosis is achieved through microscopic evaluation of the sputum or detection of gp43 antibodies. Pharmacological treatment, despite being aggressive and considering that the disease usually occurs in immunologically compromised patients, could have a favorable impact on the reduction of complications and mortality in this type of patients.