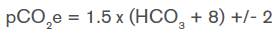INTRODUCTION
Metabolic acidosis is one of the most common conditions in patients admitted to the emergency room. In cases of normal anion gap metabolic acidosis, the most common causes are renal tubular acidosis, diuretic use, ureteroenterostomy, pancreatoenterostomy and acetazolamide or topiramate overdose. The following article presents, on the one hand, the case of a patient who develops metabolic acidosis secondary to topiramate intoxication and, on the other, a brief review of the literature on the subject.
CASE PRESENTATION
17-year-old female patient from Mexico City, residing at 2 250 m.a.s.l., currently unemployed and attending secondary school. The girl was found confused in her room, with spontaneous breathing, a lit cigarette and alcohol breath. The relative reported that she had taken 40 tablets of quetiapine, 20 of paroxetine and 20 of topiramate. She was last seen in normal neurological condition 5 hours before the incident.
The patient reported a history of major depressive disorder diagnosed 18 months earlier and attempt of autolysis, causing upper limb wounds with secondary hypovolemic shock, which required hospitalization for 20 days without admission to intensive care. Outpatient management was indicated with topiramate 50mg every 8 hours, paroxetine 20mg every 24 hours and quetiapine 100mg every 24 hours, with poor adherence. The patient indicated occasional tobacco consumption, alcohol intake of unquantified grammage, on a regular basis for a year, without drug use. She also reported menarche at age 14, irregular cycles and unknown date of last menstruation.
Physical examination showed blood pressure of 90/50 mmHg, mean blood pressure of 63 mmHg, heart rate of 100 beats per minute, respiratory rate of 30 breaths per minute, SpO2 of 89%, Glasgow Scale of 3/15, brain stem reflexes, mydriatic pupils, intercostal retractions, rhythmic heart sounds, rhonchi and bilateral basal rales, abdomen with diminished peristaltic-wave contractions, soft and depressed, without masses or peritoneal irritation data, and without pathological reflexes or murmurs. Paraclinical tests were requested and the results are presented in Table 1.
Table 1 Paraclinical tests.
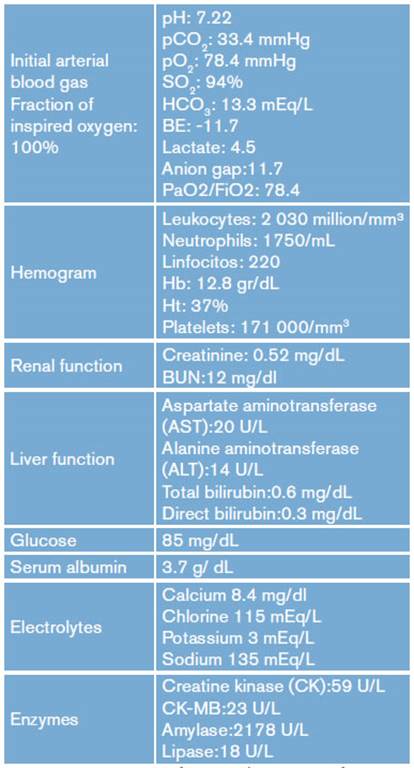
AST: aspartate aminotransferase; ALT: alanine aminotransferase; CK: creatine kinase
Source: Own elaboration.
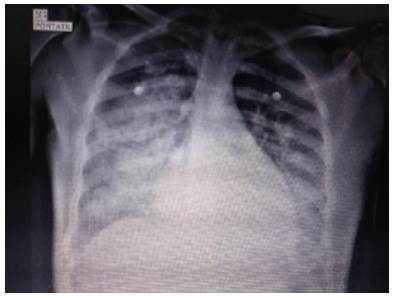
Chest x-ray showed bilateral alveolar opacities from the hilum towards the pleura in the lower two thirds of both lung fields, without images compatible with pleural effusion (Figure 1).
The electrocardiogram recorded a frequency of 91 per minute, 30° axis, PR interval of 0.16 seconds, widened QRS complex (0.20 seconds) and prolonged QT interval (0.50 seconds); no ST segment alterations or branch block were observed.
Given the patient's neurological status, rapid intubation sequence was performed in the emergency room; she presented a convulsive crisis with generalized tonicclonic seizure, controlled with diazepam 5mg IV. Arterial blood gases on admission showed mixed disorder: normal anion gap metabolic acidosis plus acute respiratory acidosis associated with hyperlactatemia type A; said hyperlactatemia is considered secondary to hypoperfusion subsequent to initial hypotension.
Neurological deterioration and electrocardiographic alterations secondary to overdose with antidepressant and topiramate were presumed in this patient. She also presented with hypotension and tachycardia attributable to the cardiovascular effects of drug overdose; in this case, despite the use of serotonin reuptake inhibitor, the patient did not show signs of serotonin syndrome.
Regarding the lungs, oxygenation disorder was diagnosed due to gasometry findings and radiographic images compatible with acute respiratory distress syndrome (ARDS). Management with crystalloids, cathartics, activated charcoal (1 g/kg) by nasogastric tube and sodium bicarbonate infusion at 1 mEq/kg was initiated, considering acidemia secondary to intoxication.
The patient was taken to the intensive care unit where hemodynamic condition improved without requiring vasopressors; lactated ringer's crystalloid with potassium chloride 2 mEq/ hour was administered for management of mild hypokalemia. Invasive mechanical ventilation was initiated based on a diagnosis of ARDS due to radiological signs and PaO2/FiO2 ratio alteration. Possible bronchoaspiration was considered due to the compromise of the state of consciousness, which also conditioned ventilatory deterioration. She also presented difficulty in ventilatory coupling requiring neuromuscular block and deep sedation.
Control arterial blood gases showed persistent normal anion gap metabolic acidosis within the first 24 hours, which was corrected subsequently (Table 2). Control electrocardiogram was performed without vasopressor or inotropic support, as well as QRS complex line and normal QT interval, maintaining sinus rhythm. Renal function was preserved without seizures; ventilatory function improved when sedation was discontinued without neurological sequelae, tolerating extubation on the fifth day. Finally, she was assessed by psychiatry, and management with paroxetine and quetiapine was restarted. The patient was discharged after 10 days without sequelae.
Table 2 Gasometric evolution during the first 24 hours*.
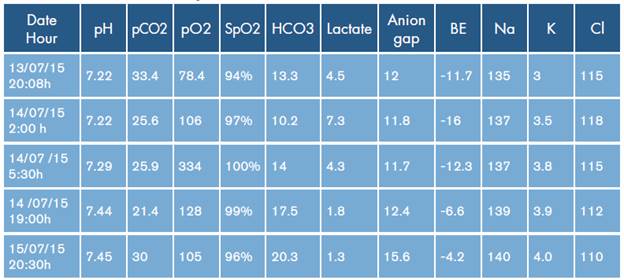
* Reference values for arterial blood gases in Mexico City: altitude: 2 250 m.a.s.l.; pH: 7.41±0.03; PaO2:62.8±4.3 mmHg; PaCO2: 35.2±4.7 mmHg; HCO3: 22±2.5 mEq/L; SO2: 91.6±4%; BE: 0±2.
Source: Own elaboration based on Vázquez-García & Pérez-Padilla 1.
DISCUSSION
Acid-base homeostasis is essential for the maintenance of life and is maintained mainly by the bicarbonate/carbonic acid buffer system interaction, which is characterized by the presence of an acid as a donor and a base as an acceptor of hydrogenions. Physiologically, a primary change in the partial pressure of CO2 causes an adaptive response in bicarbonate concentration and vice versa, while major changes lead to alterations in the acid-base balance. 2
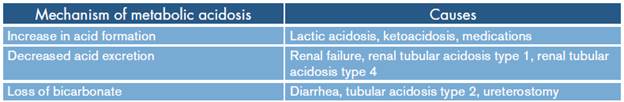
Metabolic acidosis is the most frequent acid-base alteration, caused by the increase in the concentration of hydrogenols or by the decrease of bicarbonate; these situations are conditioned by increased formation or alteration in the excretion of acids or by loss of bicarbonate as shown in Table 3. 2
Respiratory compensation should be determined in the presence of metabolic acidosis; Winter's formula is used to know if the expected pCO2 has been reached. 3
If the result is greater than expected, then a decompensated metabolic acidosis with respiratory acidosis is established; if it is lower, it refers to decompensated metabolic acidosis with respiratory alkalosis. 2-4
In general, in all acid-base disorders, compensatory responses that try to maintain pH normality and follow predetermined patterns are observed. Inappropriate compensations, by excess or defect, imply the existence of a mixed acid-base disturbance. 5
ANION GAP
In 1939, Gamble stated that the principle of electroneutrality required that the positive and negative charges in serum must be balanced. Anion gap represents a variety of "unmeasured" anions such as albumin, phosphate and sulfate 6, and is calculated using the equation 2-4
It excludes potassium anion because its value is relatively low with respect to the other ions.
Albumin is an anion and, unlike the other anions, it can be measured and fluctuate significantly in several diseases; consequently, it has been included to determine the anion gap. 6
Hypoalbuminemia causes a decrease in the anion gap. In hypoalbuminemic patients with metabolic acidosis, the anion gap may be low or normal, so the calculation should be corrected for albumin concentration in order not to underestimate the magnitude of its increase in acidosis due to the gain of non-chlorinated organic and inorganic acids. For each gram of decrease in albumin concentration, 2.5 are added to the anion gap that has been calculated. (6) Using the Figge formula, the anion gap corrected for albumin is AGAP + {0.25 x (4.4-albumin [g/dL])}. The normal anion gap values are 12 ± 4 mmol/L. 4
The main causes of high anion gap metabolic acidosis are ketoacidosis, methanol, acetylsalicylic acid, L-lactate, D-lactate, glycoles (ethylene and propylene), 5-oxoproline (associated with chronic use of paracetamol), paraldehyde, ethylene glycol and chronic kidney disease. 7
Normal anion gap metabolic acidosis
Hyperchloremic acidosis is caused by the inability to excrete hydrogen bonds or by the loss of bicarbonate ions. 3 In normal anion gap acidosis, organic acids are not formed, so the calculation of the urinary anion is measured to determine whether the etiology is renal with positive urinary anion gap or non-renal with negative urinary anion gap. The most common etiologies related to potassium concentrations are shown in Table 4.
Table 4 Causes of normal anion gap metabolic acidosis.
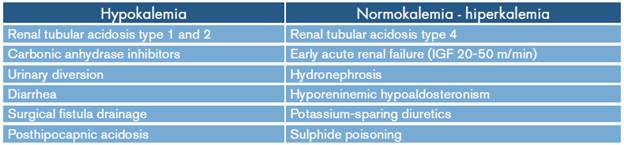
Source: Own elaboration based on Rice et al.3.
Approved in 1996 as a second-line an-ticonvulsant and prophylactic medication for migraine attacks, topiramate is a little-known cause of normal anion gap metabolic acidosis. 3 The mechanisms of action are blockade of sodium channels, potentiation of gamma aminobutyric acid (GABA), glutamate acuity and inhibition of carbonic anhydrase.
Carbonic anhydrase catalyzes the ionization of carbon dioxide to form carbonic acid, a reaction that develops continuously in the absence of the enzyme, but slowly in its presence. Balance is reached in less than 1 second, producing a proton (H+) and a bicarbonate anion. 3,4 Carbonic anhydrase is divided into 4 subgroups and 16 isoenzymes, which have a wide tissue distribution; therefore, their kinetic properties differ depending on the tissue where they are located. 8
Topiramate has a sulfa fraction that binds to the zinc ion of carbonic anhydrase; this isoform of anhydrase is determinant in bicarbonate reabsorption in the proximal tubule and in acidification in the distal tubule. 5 Topiramate shares a very similar molecular morphology with acetazolamide; the latter is the reference drug for the inhibition of carbonic anhydrase. 9,10
Carbonic anhydrase isoforms II, IV and XII are the most prevalent in the kidneys. 8 Topiramate is a high-potency inhibitor of type II, moderate potency inhibitor of type IV and mild potency inhibitor of type XII. The sulfamide analog present in this drug may be responsible for inhibiting the isoform II. 11,12
In this context, the polymorphism of type XII anhydrase is studied as a target in the formation of renal tubular acidosis. 8
Mirza et al.9 state that susceptibility to develop acidosis is caused by genetic differences, finding two single nucleotide polymorphisms known as rs2306719 and rs4984241 in the gene of carbonic anhydrase type XII, which is significantly associated with lower values of serum bicarbonate.
Renal tubular acidosis induced by topiramate
Renal tubular acidosis is characterized by limited excretion of hydrogen ions in the kidney and the reabsorption of urinary bicarbonate. 10 In renal tubular acidosis type 1 (distal), the secretion of hydrogen bonds through the collecting tubule is limited, and therefore a low concentration of bicarbonate in plasma is found. 13 On the other hand, in renal tubular acidosis type 2 (proximal), there is inability to absorb bicarbonate in the proximal convoluted tubule and 85% of the filtered bicarbonate is reabsorbed there. 13
In the context of topiramate intake, metabolic acidosis is associated with alkaline urine, positive urinary anion gap, b2 microglobinuria with serum bicarbonate, urinary citrate, low urinary pH and urinary bicarbonate, and increased fractional excretion of bicarbonate. 14
These findings are related to mechanisms of proximal and distal renal tubular acidification, that is, a combination of renal tubular acidosis type 1 and 2 that some authors call mixed or tubular renal tubular acidosis. 14
Reabsorptive limitation in renal tubular acidosis type 2 is the result of a large number of molecular defects, for example, a defect in the co-transporter Na-HCO3 gene (SLC4A4). On the other hand, alteration in the secretion of hydrogen ion in renal tubular acidosis type 1 is due to several conditions in which there is a functional alteration of the expression of the exchanger gene Cl-HCO3. 15
Other reviews state that renal tubular acidosis type 4 can be caused by limited sodium reabsorption and decreased secretion of hydrogen and potassium in the distal tubule leading to normal anion gap metabolic acidosis, hyponatremia, hyperkalemia and low urinary ammonium concentrations. 3,7 This condition occurs in patients with obstructive uropathy, interstitial tubule diseases, diabetic nephropathy and users of potassium-sparing diuretics. It is common to see a slight decrease in the concentration of bicarbonate, but there are reports in the pediatric and adult population of concentrations of 10 mEq/L. 3,8 The differences between the different types of renal tubular acidosis are summarized in Table 5.
Table 5 Characteristics of the types of renal tubular acidosis.
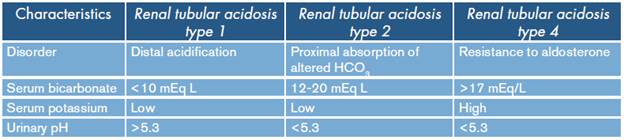
Source: Own elaboration based on Rice et al.3.
Some studies have shown that topiramate reduces serum bicarbonate levels. A study in 54 patients taking topiramate showed that 48% had metabolic acidosis with an average bicarbonate concentration of 18.8 mEq/L (range 13-21mEq/L). 16
Topiramate, besides being a potential generator of metabolic acidosis, can also cause central neurogenic hyperventilation, perhaps due to its inhibitory effect of carbonic anhydrase in the brain and subsequent CSF acidosis. 17
Possible adverse effects of metabolic acidosis include hyperventilation, fatigue, nausea and anorexia, all reported in case series of topiramate-induced metabolic acidosis. The most severe cases may include respiratory failure, heart failure and coma; however, no correlation has been found between adverse effects and drug dosage. 7 Respiratory failure has been reported in a patient with therapeutic dose intake requiring mechanical ventilation. 18
While status epilepticus, hypotension, severe metabolic acidosis and coma have been reported in overdose, neurological alterations may occur not only as a consequence of the adverse reaction to topiramate, but because of the association with valproic acid and benzodiazepine drugs. 19,20
Case reports have shown that acute metabolic acidosis attributed to topiramate has been reversed between 6 and 30 days after drug intake has been suspended. 21,22
A study in children receiving therapeutic doses between 8.2 mg/kg and 26 mg/kg found that metabolic acidosis persisted even 6 months after the initiation of topiramate treatment. This chronic acidosis can manifest itself with delayed growth, headache, diarrhea, hyperventilation, osteomalacia and osteoporosis in the adult population. 22,23
Renal clearance mechanisms are activated leading to renal hypertrophy and accelerating renal failure in patients with previous kidney disease, promoting nephrocalcinosis and nephrolithiasis secondary to the difficulty of the renal system of acidifying urine. 10,19 In addition, chronic metabolic acidosis contributes to the generation of hypoalbuminemia, as well as to the decrease in insulin resistance; these effects can even be seen in mild metabolic acidosis. 24
Other adverse effects reported as secondary to the intake of topiramate are nausea, ataxia, fatigue, weight loss, open-angle glaucoma and psychiatric symptoms such as hallucinations in 1.5-6.3% of cases. 19,25
The initial treatment is the use of activated carbon, which has shown a good in vitro adsorption effect. The use of hemodialysis is reserved for severe metabolic acidosis and hypotension refractory to initial treatment. 19
In this case, the patient was admitted with deteriorated state of consciousness associated with the consumption of quetiapine, topiramate and paroxetine, with hemodynamic instability that led to systemic hypoperfusion and subsequent hyperlactatemia. Stabilization of vital signs was achieved with crystalloid fluids. The patient also presented with adequate SpO2; however, she developed early oxygenation disorder with radiological signs of ARDS secondary to possible bronchoaspiration, which had a favorable evolution after 5 days of mechanical ventilation. At the cardiovascular level, prolongation of the QT segment was considered acquired and probably secondary to the consumption of quetiapine in association with antidepressants; this temporary condition did not generate electrical instability or conversion to torsade de pointes.
The presence of a mixed base-acid disorder with normal anion gap metabolic acidosis and associated respiratory acidosis is particularly striking since topiramate, besides inducing hyperchloremic metabolic acidosis, also causes central neurogenic hyperventilation by inhibiting carbonic anhydrase as previously discussed. The initial pCO2 level was expected to be low; however, when applying Winter's formula, it was found that the pCO2 level was above as a result of respiratory compensation in the presence of metabolic acidosis. Therefore, this sign indicates that there was respiratory depression attributable to the intake of the other medications used and that said depression could suppress the respiratory compensatory effect secondary to the metabolic acidosis produced by topiramate.
Despite fluid therapy and the management of topiramate overdose, the acid-base status was normalized after 24 hours. Management with bicarbonate was initiated considering the widening of the QRS complex and hyperchloremic metabolic acidosis, which, according to the Stewart model, is explained by the decrease in the difference of strong ions whose main components are sodium and chlorine and whose value must range between 40 mEq/L and 44 mEq/L. Bicarbonate allows the stabilization of the donation of the water molecule in the formation of radical hydrogenions and, in addition, can reverse the membrane depressant effects by increasing the extracellular concentrations of sodium and by direct effect of pH on fast sodium channels.
The most probable cause of normal anion gap metabolic acidosis in this case may be the intake of topiramate, which inhibits carbonic anhydrase, generating renal tubular acidosis type 1 due to the inability to secrete hydrogenolions in the collecting tubule and the limitation of the bicarbonate reabsorption in the proximal tubule. There was no elevation of azotees or transaminases and leukopenia, at the expense of lymphocytopenia; this was correlated with the intake levels of quetiapine.
CONCLUSIONS
Topiramate causes normal anion gap metabolic acidosis by inhibiting carbonic anhydrase. This condition occurs even at therapeutic doses. Genetic influence has been identified as an important factor for metabolic acidosis by topiramate. Regarding treatment, bicarbonate, cathartics and activated charcoal are used.