INTRODUCTION
Refeeding syndrome (RS) is a potentially deadly acute metabolic disorder that takes place during nutritional repletion in patients with prolonged malnutrition or starvation. RS commonly occurs with all types of nutritional support, but the risk seems to be higher in patients fed with enteral or parenteral nutrition. 1-3 In addition, it encompasses a set of fluid and electrolyte imbalances that affect multiple organ systems, including neurological, cardiac, hematologic, neuromuscular, and pulmonary functions. 2 Hypophosphatemia is the predominant characteristic of RS and is observed in more than 95% of cases; it also explains a large part of the symptoms of this clinical picture. 4 Other metabolic changes, such as hypomagnesemia, hypokalemia, fluid balance disturbances, and vitamin deficiencies, may also play an important role. 1
This condition is usually underdiagnosed as it lacks uniform criteria for diagnosis, while symptoms may be wrongly attributed to other clinical diagnoses. 1,5 Reports on critically ill patients show, for example, that the incidence of refeeding hypophosphatemia ranges from 34% to 52%. 1
In 2006, the UK National Institute of Health and Clinical Excellence (NICE) published recommendations for the detection and management of patients at risk of RS. 6 Some studies 7-9 have evaluated the practices and opinions of health professionals with respect to these recommendations, concluding that some aspects of the risk criteria and feeding initiation doses suggested by NICE have been adopted, although this is not the case worldwide. 8,9 For some professionals, the recommendations are too conservative and are an obstacle to providing adequate nutrition, while others think that they lead to increased costs due to the need for more frequent biochemical analyses, increased electrolyte replacement rates and closer monitoring as suggested by NICE. 7
RS prevention should be the main guideline when initiating nutritional management. The identification of patients at risk, the establishment of adequate nutritional support, and follow-up may potentially reduce the morbidity and mortality rates associated with the syndrome. 3 The description of this case intends to expose identification strategies and nutritional management of a patient at risk of RS.
CASE PRESENTATION
48-year-old male patient, mestizo, from the northern sub-region of Antioquia (Colombia), with no schooling, unemployed and economically dependent on his siblings, who presented a history of neurocognitive deficit since childhood, cleft palate, heavy daily consumption of ethanol for nearly 7 years (without information on quantity) and recent consumption of antiseptic alcohol and heavy smoking (40 cigarettes a day) since childhood. The subject did not have any surgical history nor reported consumption of medications on an outpatient basis.
The patient attended a secondary care hospital in January 2018 due to a fall under the influence of alcohol. He was discharged and re-admitted 4 days later due to a con-fusional state and weakness of lower limbs. His neurological condition deteriorated and had convulsive episode 5 days after re-admission; he was subsequently intubated and transferred to a tertiary referral hospital.
The subject was referred with a heart rate of 107 beats/minute, blood pressure of 167/110 mmHg, respiratory rate of 18 breaths/minute, temperature of 36.5°C, 99% SaO2with ventilatory support, 3/15 Glasgow with midazolam and fentanyl infusion, 3mm isocoric reactive pupils and present stem reflexes. On physical examination the subject was hydrated, with macrocephaly and sarcopenia and no other relevant findings. He entered the intensive care unit (ICU) for ventilatory support and neurological surveillance.
Upon questioning, his relatives stated that the patient had a maximum of one meal per day as he preferred to consume alcohol instead of food and that he had experienced chronic and severe weight loss (approximately 10kg) during the last year; his weight on admission was 45kg, height 155cm and body mass index (BMI) of 18.7 kg2. According to the nutritional screening tool NRS 2002, the patient had a score of 5, which indicated that he was at nutritional risk; he had a score of 2 for the item 'nutritional status', corresponding to BMI between 18.5 and 20.5 kg/m2, plus deterioration of the general state or energy intake of 25-60% in the last week, and a score of 3 for the item 'disease severity' since he was a critical patient at the ICU. He was diagnosed with severe malnutrition by the ICU dietitian nutritionist.
The initial paraclinical tests showed chronic hepatopathy by ethanol, without cirrhosis or alcoholic hepatitis on abdominal ultrasound. The paraclinical tests on admission were creatinine: 1.21 mg/dL; urea nitrogen: 49 mg/dL; sodium: 148 mmol/L; potassium: 4.28 mmol/L; calcium: 8.7 mg/dL; chlorine: 11 2.5 mmol/L; aspartate transaminase: 78 u/L; alanine aminotransferase: 207 u/L; alkaline phosphatase: 192 u/L; gamma-glutamyl transferase: 223 u/L; direct bilirubin: 0.78 mg/dL; total bilirubin: 0.94 mg/ dL; hemoglobin: 15 g/dL; hematocrit: 45.1%; magnesium: 2.81 mg/dL; folic acid 2.6 ng/mL; and vitamin B12: 333 pg/mL.
Computed tomography (CT) of the skull showed noncommunicating hydrocephalus, subarachnoid hemorrhage and chronic subdural hygromas without indication of surgical intervention and probably unrelated to the current neurological condition. In addition, status epilepticus was ruled out in electroencephalogram, so it was decided to manage Wernicke-Korsakoff encephalopathy by starting thiamine 200mg intravenously every 8 hours and lorazepam 1mg every 8 hours due to risk of abstinence.
At 24 hours after admission to the ICU, enteral nutritional support was initiated using a gastric tube with the lactose-free polymer formula available in the hospital (protein: 14%, carbohydrates: 66%, fat: 20%, sodium: 84 mg/100mL, fructooligosaccharides: 1g/100mL). It was established that the patient had a high risk of suffering RS according to the NICE criteria (Table 1) 6 and the personal history described above (alcohol abuse, inadequate food intake for more than five days, loss of approximately 18% of weight in the last year and BMI in the lower limit of normality). For this reason, early nutritional support was initiated with maximum 15 kcal/kg, maintaining stable caloric intake the first 3 days and then increasing between 5 and 15 kcal/kg/day, in addition to follow-up laboratory tests during the first 72 hours of nutritional support.
Table 1 Criteria to determine people at high risk of developing refeeding problems BMI: body mass index.

Source: Own elaboration based on NICE criteria
Before starting enteral nutrition, baseline paraclinical tests were taken to monitor RS, being within the normal range: phosphorus: 3 mg/dL (0.97 mmol/L), magnesium: 2.55 mg/ dL (1.06 mmol/L) and potassium: 3.6 mmol/L. Then, enteral nutrition was started with a caloric intake of 14 kcal/kg and 13 mL/kg of current weight; this was maintained for the first 3 days of nutritional support. Control paraclinical test taken the day after initiating enteral nutrition showed that the phosphorus value decreased to 2 mg/dL (0.65 mmol/L), without the need for repletion. On the third day of nutritional support, phosphorus was again within the normal range (Figure 1). Regarding potassium, it decreased to 3.3 mmol/L, and was replaced with 0.8 mmol/ kg/day; during the following days, it was within the normal range. Magnesium values remained normal during all days of nutritional support.
On day 4, the caloric intake increased to 22 kcal/kg and 21 mL/kg, leading to establish a management plan that consisted of reaching the nutritional goal of 25 kcal/kg on day 5 or 30 kcal/kg on day 7 based on the clinical condition of the patient. In addition, since the first day of nutritional support, the patient received folic acid 5mg every 24 hours and pyridoxine 50mg orally every 24 hours. While fed with enteral nutrition, the subject did not present any gastrointestinal symptoms related to nutrition intolerance or adverse events with the nutritional treatment implemented.
In order to assess his neurological condition, sedation was suspended 24 hours after admission to the ICU. During the following 3 days, the patient presented eye response without contact with the environment or motor response. He also reported left mydriasis, hypertension and bradycardia. CT scan of the skull showed extensive acute ischemic changes in the right cerebral hemisphere and cerebral edema, so hyperosmolar therapy was initiated.
The patient showed greater neurological deterioration on the fifth day of stay in the ICU. Shows an adaptation For this reason, due to his baseline condition and the last identified changes, the subject was considered irrecoverable and minimal baseline support was offered, while the objective of management was redirected to palliative care. The patient died on the sixth day of hospitalization.
DISCUSSION
Although RS is a condition known since the end of World War II and is defined as a life-threatening condition, early detection of risk factors and the implementation of measures to prevent it are not common in the nutritional care process. 7,8 One of the reasons may be that nutritional recommendations emphasize on avoiding high rates of malnutrition: it is reported that the prevalence of malnutrition in critically ill patients can range from 37.8% to 78.1%. 10 In this regard, the concern of health professionals to avoid undernutrition, seek early nutrition, not delay the achievement of nutritional goals, among others, prevails over the concern to implement gradual nutritional treatments in patients at risk of RS.
Another reason is that risk factors for developing RS may be vague. The literature reports very varied and common risk factors among patients admitted to a hospital 2,5,6,11; therefore, there is concern that implementing RS risk prevention measures could put patients at greater nutritional risk, increasing the number of patients who experience a delay in meeting their nutritional goals.
A third reason is the lack of a clear definition of RS, which leads to underdiagnose it and cast doubt on the true relevance of implementing strategies to avoid it. A systematic review including 45 RS-related studies conducted between 1989 and 2015 shows heterogeneous definitions; most studies included hypophosphatemia in their definition, either as a cut-off point or as a relative change from baseline. 5 As it does not have standard criteria for diagnosis, RS goes unnoticed and is attributed to other types of situations such as medical management, basic pathology, clinical complications and drugs action.
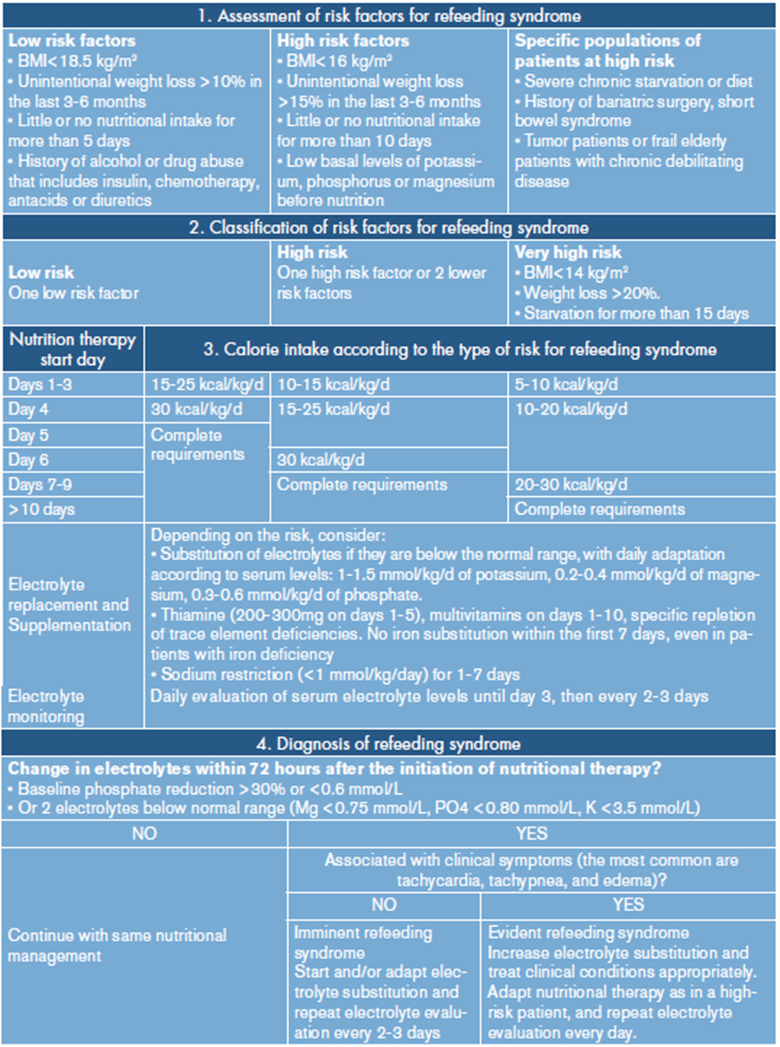
One way to avoid imprecision in the classification of RS risk, and thereby prevent over-or underestimation of patients at risk, is the development of care guidelines. The Hospital Pablo Tobón Uribe of Medellín does not have an adult patient RS management guide, although it recognizes the importance of this condition and is working on a care protocol. While own guidelines are generated, the guidelines for RS risk assessment and nutritional management by NICE: nutrition support for adults (Table 1) 6 and the Friedli et al. consensus (Table 2) are being used 3 for RS in inpatients.
This last consensus has the added value of detailing the management that should be offered depending on the type of refeeding risk of each subject; patients are classified as low risk, high risk and very high risk 3. This classification could help not to generalize and not to fall into the error of delaying nutritional goals in patients who do not require it.
For the case reported here, nutrition was started at 30 mL/hour, which provided 14 kcal/ kg and remained the same for the first 3 days of nutritional support. Although the NICE guidelines usually suggest not exceeding 10 kcal/kg, the expert consensus proposed between 5 and 15 kcal/kg depending on the risk classification. This patient was classified as high risk, a group for which it is recommended not to exceed 10 kcal/kg to 15 kcal/kg during the first 3 days of nutritional support. Some practitioners believe that calorie restrictions become an obstacle to providing adequate nutrition 7; however, a multicenter randomized clinical trial of patients with phosphorus depletion <2 mg/dL (0.65 mmol/L) within 72 hours of initiation of nutrition determined that calorie restriction at 20 kcal/hour for at least 2 days appears to be an appropriate therapeutic option for critically ill adults. This restriction led to significant improvements in the overall survival time and in the reduction of mortality at day 60 of follow-up. 12
On the other hand, Friedli et al.3 also propose an algorithm that allows tracking patients at risk and diagnose and treat RS, if applicable. In the reported case, the patient presented phosphorus depletion of over 30%, which is consider by some as a criterion for diagnosing RS; however, the patient did not present severe hypophosphatemia or clinical manifestations, and on the third day of nutritional support the serum phosphorus was normalized without the need for repletion or changes in caloric intake. Therefore, according to the algorithm, this was not considered as RS.
Table 2. shows an adaptation of the work algorithm proposed by Friedli et al.3, which could serve as a guide for health institutions to assess the risk of refeeding and in the nutritional treatment according to the type of risk.
CONCLUSIONS
At the beginning of the nutritional treatment, the patient presented a decrease of phosphorus of more than 30% with respect to its basal value; however, serum phosphorus normalized after maintaining a stable caloric contribution during the first three days of nutritional therapy. Apparently, the patient did not develop RS and the nutritional management offered at all stages (from the detection of RS risk to the implementation of enteral support and follow-up) was successful in preventing the onset of this condition and its complications.
One of the limitations of this case report was the difficulty in accessing anthropometric variables and food background information. Due to his critical condition, the patient was unable to provide information, the anthropometric variables were difficult to establish and his family was unable to provide exhaustive information on food consumption. The subject was classified at high risk of developing RS, but key variables - such as dietary anamnesis- were not obtained, which if known, could have pointed to a greater risk of refeeding and lower initial energy needs.
Prevention is the main recommendation for avoiding the onset of RS and associated complications. It is important to recognize when a patient is at risk, provide adequate management, and monitor the patient to prevent the syndrome from developing. Sometimes it is not possible to prevent RS from happening, but it is possible to prevent the patient from developing serious complications or a fatal outcome. All health institutions should establish the assessment of the risk of refeeding syndrome in their care protocols, as well as a nutritional treatment according to the type of risk.
There is still a wide array of definitions, reported incidence rates, preventive measures and treatment recommendations for RS; therefore, more high quality prospective research is needed to fill this gap.