INTRODUCTION
Histoplasmosis is an opportunistic fungal granulomatous infection with higher prevalence in tropical regions, mainly in Central and South America. It is caused by inhalation of microconidia of Histoplasma capsulatum var. capsulatum, a dimorphic fungus that develops in soil and plant debris, especially in areas with high nitrogen content usually associated with caves and bird droppings. 1
Histoplasmosis has a wide range of clinical presentations that depend on three factors mostly: fungal load, virulence and histoplasma strain, and host immune status. 2 In addition, it can vary from asymptomatic patients with a less severe state to severe infections in the context of hematogenous dissemination or through the reticuloendothelial system, more frequently involving organs such as liver, spleen, bone marrow and skin. 3
In patients with human immunodeficiency virus (HIV) infection, the most frequent clinical presentation is the progressive disseminated form, which usually has an acute or subacute course. 4
Peritonitis associated with H. capsulatum is extremely rare, with few cases reported in the literature. Risk factors for developing fungal peritonitis include prior use of antibiotics, immunosuppression status, environmental exposure, intra-abdominal surgery, and extra-peritoneal spread of fungal infection. 5 The following is a case report of a patient with HIV infection, systemic lupus erythematosus with lupus nephritis, and chronic kidney disease on hemodialysis diagnosed with peritoneal histoplasmosis (PH).
CASE PRESENTATION
Male patient of 44 years of age, from Río Viejo (Bolívar, Colombia) and resident in Bogotá D.C. for 3 years in a household classified as socioeconomic stratum 2 in the locality of Engativá; he was unemployed for the last year and previously worked as a heavy machinery operator. He had been diagnosed with HIV infection C3 category and AIDS (clinical stage 4), with a CD4 count of 416 cells/mm3 and a viral load of 36,645 copies/mm3, treated with anti-retrovirals including abacavir 600 mg/day, lamivudine 50 mg/day and dolutegravir 50 mg/ day. He also had a history of systemic lupus erythematosus with lupus nephritis, and stage 5 chronic kidney disease previously treated with mycophenolate mofetil 500 mg/day and on renal replacement therapy with peritoneal dialysis.
The patient attended consultation due to a clinical picture of three months of evolution consisting of abdominal distention, early prandial satiety, nausea, anorexia and progressive dyspnea. The subject assisted to outpatient consultations on multiple occasions and was diagnosed with possible tense ascites, for which an evacuation and diagnostic paracentesis with conventional ascitic fluid studies was scheduled. No infectious etiology was documented in peritoneal liquid cultures (negative for common germs and fungi) or Ziehl-Neelsen staining (no acid-alcohol resistant germs observed); adenosine desaminase was reported as normal (data not available in clinical history) and there were no other relevant findings in basic studies of ascitic fluid.
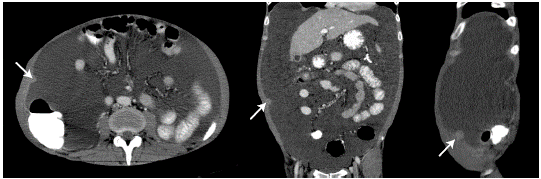
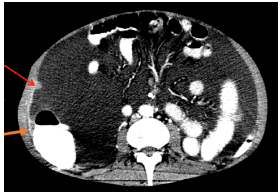
On physical examination, the patient was in poor general condition, tachycardic, normotensive, afebrile and with cachexia; his weight was 48kg, and he presented with distended abdomen with shifting dullness, cardiopulmonary auscultation without alterations, positive ascitic wave and pain on palpation without signs of peritoneal irritation, and peritoneal dialysis catheter without local inflammatory signs. A computed tomography (CT) of the abdomen was performed, showing a single nodular image of well-defined edges, with soft tissue density, of 12mm in diameter, located in the peritoneal plane of the right abdominal wall and associated with abundant ascites of free characteristics, splenomegaly and slight thickening of the parietal peritoneum (Figure 1 and 2).
Source: Document obtained during the study.
Figure 1 CT scan of the abdomen, axial, coronal and sagittal planes, showing nodular lesion (arrow) in the parietal peritoneum.
Source: Document obtained during the study.
Figure 2 CT scan of the abdomen, axial plane, showing thickening (orange arrow) and nodular lesion (red arrow) in the parietal peritoneum.
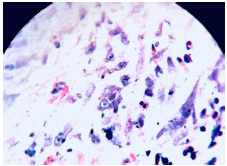
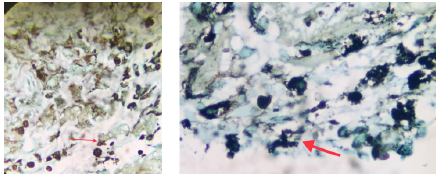
Subsequently, a diagnostic laparoscopy with biopsy of the peritoneal lesion was performed, revealing fibroconective stromal tissue with cells of reactive aspect; hemorrhagic and necrotic foci, and infiltrate of eosinophils, lymphocytes and, to a lesser extent, plasmocytes. No granulomas or tumor cells were obtained. Special stains were performed with findings in Grocott-Gomori's methenamine silver stain suggestive of histoplasma immersed in necrotic tissue (Figures 3 and 4).
Source: Document obtained during the study.
Figure 3 Hematoxyline-eosine staining of fìbroconective stromal tissue with reactive cells, eosinophil infiltrates, and hemorrhagic and necrotic foci.
Source: Document obtained during the study.
Figure 4 Grocott-Gomori's methenamine silver stain suggestive of histoplasma forms (red arrows) in a small necrotic tissue fragment.
In addition, fungi were cultured in a peritoneal sample at 6 weeks, which confirmed the diagnosis of H capsulatum infection. Peritoneal dialysis catheter was removed, renal support was initiated with hemodialysis, as well as in-hospital treatment with amphotericin B de-oxycholate 50 mg/day in continuous infusion for 15 days (indicated by the Infectious Disease Department for patients with chronic kidney disease on hemodialysis).
During the diagnostic approach, pulmonary involvement was ruled out by chest CT and negative blood cultures. During hospitalization follow-up, the nutrition service diagnosed him with severe protein-calorie malnutrition, thus conditioning an additional factor for immunocompromise that was managed with enteral nutritional support with satisfactory clinical response.
The treatment did not have any adverse effect and no adjustment of the dose scheduled for in-hospital management of amphotericin B de-oxycholate was required. Once the amphotericin B scheme was completed with good tolerance, and considering his clinical improvement without recurrence of ascitic syndrome and tolerance to enteral nutritional support weaning and the oral route, it was decided to continue outpatient management with itraconazole 200mg every 1 2 hours indefinitely, according to the concept of infectology. Control appointments were scheduled with nephrology, clinical nutrition, infectology and HIV program to continue with multidisciplinary outpatient management.
The patient was considered to have a good prognosis due to guided management of specific etiology, a multidisciplinary evaluation plan for physical rehabilitation and nutrition, and the management of comorbidities.
DISCUSSION
This report presents a case of peritoneal infection by histoplasmosis in a patient with HIV infection, systemic lupus erythematosus and severe protein-caloric malnutrition. This infection is found throughout the world, and since the twentieth century, it has been considered part of the definition of acquired immunodeficiency in HIV patients. 6,7 It is also characterized by respiratory symptomatology -either acute, subacute or chronic- when it involves only the lungs, and by systemic manifestations related to its disseminated form; the latter is more frequent in the elderly and in immunocompromised patients. 8
In patients with AIDS, H. capsulatum is considered opportunistic, and is usually associated with low CD4 counts (<150 cell/ mm3), with higher incidence in patients coming from endemic areas for the disease and in patients with aspiration of a high inoculum of microconidias of the fungus. 9 Therefore, in the case described here, the risk factor was not the history of HIV infection, given that at the time of the PH diagnosis the patient had a CD4 count of 416 cell/mm3. The risk factors for DH, in this case, involved immunosuppression determinants: systemic lupus erythematosus and its treatment, chronic kidney disease and severe protein-caloric malnutrition.
The disseminated form of histoplasmosis is characterized by the onset of respiratory symptoms, subsequently accompanied by hepatosplenomegaly, adenopathies, colonic masses, chorioretinitis, central nervous system lesions and skin lesions. 8 Peritoneal manifestation is part of the spectrum of disseminated histoplasmosis (DH) presentation, while peritoneal fungal infection in immunosuppressed patients is usually caused by Candida spp; infection by H. capsulatum is rare. 10 With regard to the DH diagnosis, skin lesion examinations are usually positive in 3-55% of the cases, while antibody and urinary antigen detection is positive in 70-90% and lung culture in 5070%; sensitivity depends on the clinical form and immune status of the host. 11
In diagnostic imaging, PH is characterized by diffuse nodular thickening of peritoneal surfaces that have soft tissue attenuation on CT and are enhanced by intravenous contrast administration. Other findings may include ascites, hepatic or splenic microabscesses of miliary distribution, peritoneal or hepatic granulomas, and diffuse striation of the omentum or mesenteric fat. 12,13
Finding disseminated peritoneal lymph-adenopathies or nodules is highly relevant as they suggest peritoneal involvement secondary to hematogenous dissemination. 13 Gastrointestinal involvement (thickening of the terminal or blind ileum) by histoplasmosis suggests dissemination by contiguity secondary to intestinal microperforations. PH is included in the spectrum of granulomatous peritonitis and, therefore, its imaging characteristics are indistinguishable from peritoneal tuberculosis infection. 12
PH is a rare form of DH that has been seldom reported worldwide, with one case in South America and none in Colombia. The case described here takes on great importance as it is the first to be reported in the country and the second in South America, thus providing valuable information on this rare disease.
The first case of PH was described in 1970 by Reddy after conducting the necropsy of a woman with disseminated disease 14; the only case reported in South America was a woman of Lebanese origin without other related diseases that occurred in Venezuela in 1989. 15
Most PH reports have been associated with peritoneal dialysis 5,14-17: the first was reported by Ma 16 in 1985 and the last by Ijaz & Choudhury 5 in 2010. The present case adds to this list of infections associated with peritoneal dialysis catheter, since the patient was under management with peritoneal dialysis for chronic kidney disease due to class V lupus nephritis at the time of diagnosis. Among the diagnostic methods used for PH, Lim et al.17 cultivated H. capsulatum in the peritoneal dialysis fluid of a patient who presented signs and symptoms consistent with peritonitis. In 1993, Lopes et al.18 and Lopes et al.19, besides performing the culture of peritoneal dialysis fluid, observed fungal forms compatible with the disease on direct examination. The clinical approach presented here focuses on the invasive peritoneal study of a patient with recurrent ascites in the context of multifactorial immunosuppression, in whom conventional ascitic fluid studies failed to establish an etiology and whose abdominal diagnostic imaging showed focal lesion amenable to study.
In Colombia, histoplasmosis is frequent, especially in risk groups according to the national survey of 1992-2008, which found that 70.5% of patients with histoplasmosis had AIDS and 7% had other types of immunosuppression 20; both situations were found in the reported patient. It is important to note that PH should be treated as DH in the absence of a specific guideline for treatment 21, with a duration based on the clinical evolution and extrapolation of other types. The initial management with amphotericin B was administered in the hospital for 15 days, during which time the patient presented progressive clinical improvement; he was finally discharged with an indication of indefinite management with voriconazole and follow up by infectology to determine the suspension of the drug.
In patients with chronic kidney disease who are on renal replacement therapy, amphotericin B deoxycholate and voriconazole are used at the same doses as in patients without chronic kidney disease, without any further consideration being required. However, adverse effects to be considered in both types of patients should always be followed up.
CONCLUSIONS
PH is a rare entity that is part of the spectrum of clinical manifestations of DH and requires high clinical suspicion, especially in immuno-compromised patients. Its diagnosis should be based on the study of ascitic fluid, being invasive peritoneal study an option in patients in whom microbiological isolation in peritoneal fluid is not achieved. Because of its low incidence, treatment should be based on what is described for other forms of DH. Likewise, the clinical evolution of any hospitalized patient must be interpreted in an appropriate way, since one or several diseases may share signs and symptoms, and the course of a disease may be different from one patient to another. In short, clinical suspicion allows for proper diagnosis and, in turn, determines a comprehensive and timely management.
There are clinical settings that make difficult reaching a conclusive diagnosis, requiring unusual tests or empirical treatments, depending on each context. The present case provides information on the diagnostic approach and in-depth evaluation required by immunocompromised patients, especially those with multiple comorbidities and psychosocial risk factors.
The strength of the current clinical case lies in the fact that it was possible to achieve the definitive diagnosis of a rare entity, which also had an unusual clinical and radiological presentation. Some of the limitations are the unavailability of complete data from the previous clinical history, which included serial peritoneal fluid studies and outpatient follow-ups, given that the patient did not attend the scheduled control appointments and there was no in-person or telephone contact after hospital discharge. Moreover, it was not possible to use other methods for immunological diagnosis, molecular diagnosis and detection of fungal antigens because they were not available at the facilities of the health service provider where the patient was treated.














