INTRODUCTION
Acrodermatitis enteropathica (AE) is caused by zinc deficiency and may be inherited or acquired. The acquired form may occur due to inadequate intestinal absorption of this trace element in conditions such as alcoholism, anorexia nervosa, cystic fibrosis, celiac disease, bariatric surgery, inflammatory bowel disease and any form of chronic diarrhea. 1
Rash is usually one of the first clinical manifestations of this disease: perioral and acral lesions in the form of vesicles, blisters, pustules, honey-colored crusts and well-defined scaly plaques that later take on a psoriasiform pattern. 1-3 To date, there is no specific and sensitive biochemical indicator for the diagnosis of acquired AE (4); the diagnosis is suspected due to clinical manifestations that are sometimes accompanied by low plasma zinc levels, and is confirmed when there is improvement of the symptoms after initiating supplementation. 1,5
The treatment of acquired AE is zinc supplementation, but there is still some doubt about the dose and duration of the supplement needed. Some indicate that a dose of 15-30 mg/day is sufficient for adults 1,6, while others report the need to supplement about 3 mg/kg/day of elemental zinc. 1,7-10
The following is a case of acquired AE in an adult patient with a history of bariatric surgery and intestinal resection. The nutritional management for this patient is described below.
CASE PRESENTATION
A female patient, 46 years old, mestizo, from Medellín (Colombia), with a technical degree and unemployed for about a year, entered the emergency department due to a trauma to the chin secondary to a fall from her own height. The woman had a history of gastric bypass 10 years ago and intestinal gangrene a year and a half ago, which required intestinal resection and correction of gastric bypass surgery; during the procedure, one and a half meters of residual small intestine was left between the proximal intestinal anastomosis and the bowel. Considering her surgical history, she received 300mg of ferrous sulfate, 600mg of elemental calcium, 1 tablet of folic acid (she did not remember the amount of mg), 2000 IU of vitamin D3 plus 80mg of magnesium, and 20mg of omeprazole daily.
The patient had also a history of hypothyroidism that was being treated with levothyroxine 75 μg/day, trigeminal neuralgia surgery being managed with pregabalin 75 mg/day, bipolar II disorder, and alcohol and opioids dependence in treatment with quetiapine XR 300 mg/day, bupropion 150mg (interdia), escitalopram 20 mg/day and lamotrigine 50 mg/day. Also, 2 weeks earlier, she started valproic acid 750 mg/day due to a paroxysmal attack which was interpreted as a convulsion.
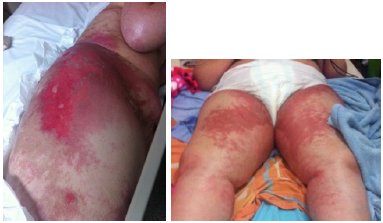
On physical examination, the patient was conscious, alert and oriented. She presented bilateral grade 3 edema in arms and legs, eroded and erythematous plates of different sizes in the lateral region of both thighs, and more livedoid lesions with dorsal involvement in the posterior region and the soles of both feet. These findings were also evident but more moderate in the buttocks (Figure 1). In addition, she presented with erythema with scaling in the vagina and labia majora and fissures in the tongue and labial commissures. During the examination, she stated that she had been presenting with the skin rash for 2 months, which began in back of her feet and later compromised thighs, oral mucosa and vagina. She also referred a burning sensation and pruritus, and liquid depositions of several months of evolution.
Clinically, toxicodermia versus zinc deficiency with acrodermatitis was suspected, so skin biopsy with pathology was scheduled for differential diagnosis. Treatment of skin lesions with emollient and epidermal barrier repairer was initiated.
While awaiting the pathology report, the patient was admitted to the intensive care unit (ICU) 5 days after her admission due to a septic shock with no clear focus and status epilepticus. Enteral nutrition was initiated by gastric tube due to the need for tracheal intubation and neurological alteration. Moreover, the patient received supplementation with vitamin C: 500 mg/every 12 hours, vitamin E: 200 IU/day, and multivitamin associated with minerals: 1 tablet/day, which included zinc 22.5mg. At that point, there were no signs of improvement in the skin lesions.
During her stay at the ICU, pathology reports were obtained (10 days after hospital admission and 5 days after ICU admission) indicating skin with hyper and confluent parakeratosis, absence of granular layer, irregular epidermal hyperplasia in some areas with psoriasiform pattern and some superficial keratinocytes of clear cytoplasm, and spongiosis. The dermis had dilated capillaries and superficial perivascular lymphocytic inflammatory infiltrate, findings that correlated with a clinical picture compatible with zinc deficiency, even though, from a histological viewpoint, the symptoms could not be differentiated from other diagnoses involving deficiency dermatitis, such as pellagra or necrolytic migratory erythema.
The treating medical team opted for a diagnosis of acquired acrodermatitis enteropathica and began treatment with enteral administration of elemental zinc 200 mg/day (3.3 mg/kg, dry weight: 60kg) for 6 days, with subsequent change to 100 mg/day intravenously for 5 days to avoid possible fluctuations in enteral absorption. Zinc supplementation improved the clinical picture with less erythematous and xerotic skin, in residual pigmentation phase, and residual desquamation. Upon completion of intravenous zinc treatment, a maintenance dose of 22.5mg of oral zinc was continued.
During her stay at the hospital, the patient said she felt sad about her health condition, with a feeling of loss of autonomy and independence, and tired due to her state and long period of hospitalization, for which she received support from the psychology service. The woman remained in the ICU for 31 days with prolonged mechanical ventilation, and was discharged after 50 days with healthy skin and indications to continue with all supplements on an outpatient basis, including 22.5mg of zinc per day.
The patient was readmitted twice (on the 20th day and on the 7th month after discharge) due to reactivation of skin lesions clinically compatible with acrodermatitis enteropathica. In the first readmission, she had serum zinc levels of 0.29 mg/L (normal value: 0.66-1.020 mg/L), so she was prescribed 78mg (1.3 mg/ kg, dry weight: 60kg) of elemental zinc per day and was indicated to continue with this same dose on an outpatient basis. The woman was hospitalized for 1 2 days.
During the second readmission, the patient received total parenteral nutrition (TPN) for severe lesions that affected the oral cavity and prevented her from eating; she was also diagnosed with intestinal failure by malabsorption associated with residual small bowel. A new skin biopsy was performed, showing, once again, histological findings compatible with deficiency dermatitis. Zinc values of 1.1 2 mg/L were obtained and the elemental zinc supplement dose was increased to 180 mg/ day (2.7 mg/kg, weight: 67kg). The patient was discharged on day 1 7 with indication to continue supplementation at home.
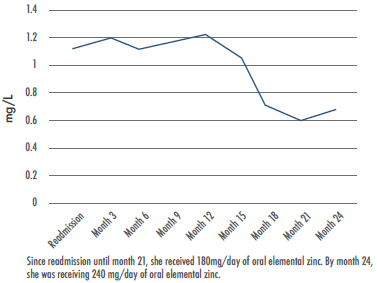
At the time of writing this paper, outpatient follow-up had been performed and the patient had been on zinc supplementation for 3 years; the dose was adjusted according to blood zinc levels and the recurrence of skin lesions (Figure 2). The last dose she received was 240mg of elemental zinc/day (2.8 mg/kg/day, weight: 84.4kg), achieving normal levels and healthy skin. No intolerance or complications associated with zinc supplementation had been observed.
DISCUSSION
Acquired AE should be suspected in adult patients with intestinal malabsorption who present dermatological symptoms. Intestinal malabsorption seems to be the main cause of zinc deficiency in this case report; a history of intestinal resection leading to chronic diarrhea with malabsorption may explain the deficiency of this trace element, which is absorbed and excreted mainly in the intestinal tract. 1
In case of clinical suspicion, it is important to analyze skin lesions and measure blood zinc levels. In the described case report, a diagnosis of acquired AE was suspected due to the clinical manifestations and histological characteristics of the biopsy, and it was confirmed based on the improvement of the symptoms after initiating the supplementation. Zinc levels in plasma were not initially measured in this case, however, in future cases, this could be an important aspect to consider because it can contribute to the differential diagnosis, since the clinical manifestations and histological characteristics of acquired AE are indistinguishable from other nutrient deficiency diagnoses such as pellagra. 1,11,12
Plasma zinc represents less than 0.2% of body reserves, thus limiting its usefulness as a method to diagnose acrodermatitis. 4,10 Zinc deficiency with normal plasma levels 13,14 have also been reported, such as the one observed in the patient's second hospital readmission when her skin lesions reactivated even though she had normal zinc levels. Measuring only the levels of this element in plasma in search of a deficiency may have limitations, but a proper correlation between clinical manifestations, histopathological findings from skin biopsy and plasma zinc levels is essential to identify the deficiency and initiate supplementation.
In the present case, supplementation was initiated with 3 mg/kg of oral elemental zinc with subsequent adjustments to the dose according to the evolution of the lesions and the values of this mineral in plasma, first monthly and then quarterly. The patient received up to 240mg of oral elemental zinc on an outpatient basis without adverse events.
In case reports of patients with a history of abdominal surgery, supplementation doses ranged from 2 mg/kg/day of venous elemental zinc to as much as 300 mg/day orally. (7-9,15,16) The recommended duration of the supplementation is not clear, since the available case reports do not specify the time of the intervention, although they show improvements of the lesions with only days or weeks of treatment. 7-9,15,16
Oral zinc intake of 8-11 mg/day or parenteral zinc of 2.5-5 mg/day is considered sufficient to meet the mineral requirements in adults; in patients with intestinal malabsorption, the requirement could increase to 12-17mg of parenteral administration per liter of fluid lost. 5,17-19 In the treatment of acquired AE, the doses of supplementation used exceed previous recommendations, so it is important to follow up on the occurrence of adverse effects related to excess zinc, such as gastric irritation, abdominal pain, nausea and vomiting. 10 Copper should also be supplemented and its plasma levels monitored because excess zinc interferes with its absorption; thus, it is generally recommended to supplement 1mg of copper for every 8-5mg of zinc. 20
The strength of this case report is the long-term outpatient follow-up received by the patient under a structured bowel rehabilitation program. The assessments were made during joint consultations that involved the bowel rehabilitation physician and the nutrition professional. This resulted in adequate control of acquired AE and
intestinal malabsorption. Limitations included not having measured plasma zinc levels from the beginning, which could have been an important aspect for differential diagnosis, and that copper levels were not included in the monthly or quarterly follow-up lab tests, although the appearance of possible adverse events due to excess zinc was monitored.
CONCLUSIONS
Health professionals should suspect acquired AE in patients with inadequate nutrient intake or intestinal malabsorption, who also have a confluence of relevant dermatological findings. For the differential diagnosis of acquired AE and for the early initiation of zinc supplementation, it is important to correlate clinical manifestations, histological findings of skin biopsy, and plasma zinc levels. In this patient with intestinal malabsorption, oral supplementation for 3 years at up to 240 mg/day was adequate for controlling the disease; follow-up through blood zinc levels and physical examination of the skin were also essential to adjust the supplemented dose.