INTRODUCTION
Worldwide, the estimated incidence of venous thromboembolism in newborns is 0.5 per 10 000 live births. 1 In these patients, the risk of thrombotic complications increases up to 40 times if this condition occurs during their first month of life. 2
Greenway et al.1 claim that about 90% of thrombosis cases in newborns are associated with the use of intravascular devices such as the central venous catheter. This happens mainly in premature babies, in whom such devices may affect the wall of the vessels, generating blood stasis, which, together with the lability of their homeostatic system, can increase even more the risk of developing clots.
Superior vena cava syndrome (SVCS) is a complication caused by the obstruction of blood flow through the superior vena cava. There is no certainty about its proper treatment, as the clinical evidence does not show which treatment option is best and at what dose medications it should be administered. Considering that this pathology is caused by a thrombus originated by the inflammatory reaction against the central venous catheter, when it occurs, anticoagulant management should be initiated and the device removed, always bearing in mind that the risk of bleeding complications in premature infants is high. 3,4
CASE PRESENTATION
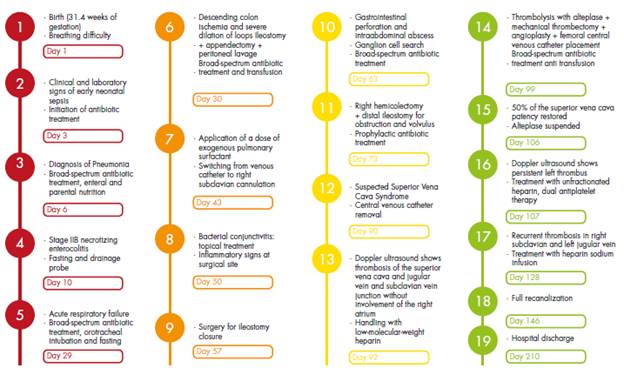
Premature male patient (31.4 weeks of gestation), Caucasian and from the municipality of Floridablanca (Colombia), who was born by cesarean section due to absence of diastole flow in the umbilical artery and unsatisfactory fetal status reported in Doppler ultrasound. The mother, blood type A+, was 28 years old and had a history of preeclampsia and a stillborn pregnancy at 26 weeks due to renal agenesis; she did not report a history of thrombophilia.
The patient, who weighted 1 365gr at birth, presented acute respiratory distress syndrome on his first day of life, requiring treatment with invasive ventilatory support and the application of a dose of exogenous pulmonary surfactant, with which he had a favorable evolution at first. However, on his third day of life, he required first-line antibiotic therapy due to clinical and laboratory changes suggestive of early neonatal sepsis; two days later, it was necessary to adjust the antibiotic regimen as imaging changes suggested pneumonia.
The child received comprehensive treatment with a mixed nutritional recovery plan (enteral and parenteral) from his second day of life until he gained weight. However, at 10 days of age, he presented stage IIB necrotizing enterocolitis, requiring access with a left central jugular venous catheter for total parenteral nutrition and second-line antibiotics. At 30 days of life, he required a surgical intervention; intestinal ischemia of the ascending colon and severe dilation of the loops of bowel were observed, so ileostomy, prophylactic appendectomy and peritoneal lavage were performed.
Due to multiple complications, at 32, 42, 56 and 82 days of age, the patient required surgery to manage a new intestinal perforation, peritonitis, intestinal obstruction due to adhesions and intestinal volvulus, respectively. Biopsies of descending and sigmoid colon were taken and sent for study on suspicion of Hirschsprung's disease but, since the results showed ganglion cells in all the samples examined, this pathology was ruled out.
Already in the convalescent phase, at 90 days of life, the patient presented a sudden increase in the diameter of the neck in the left lateral region that radiated to the left hemiface. This increase was associated with jugular distension, central cyanosis, use of accessory muscles and tachypnea, so SVCS was suspected. Consequently, the central venous catheter that had been switched from the left jugular vein to the left subclavian vein at 59 days of age was removed (Figure 1).
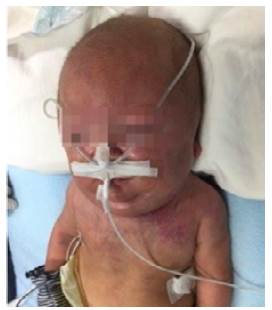
Source: Document obtained during the study.
Figure 1 Patient with increased neck diameter (90 days of life).
Due to the persistent increase in the diameter of the neck after catheter removal, a Doppler ultrasound of the neck and large vessels was requested, which revealed thrombosis of the superior vena cava without involvement of the jugular and subclavian vein junction in the right atrium. For this reason, it was decided to initiate anticoagulation with low-molecular-weight heparin considering the high risk of paradoxical pulmonary thromboembolism caused by the persistence of the patent foramen ovale. Likewise, due to the active symptomatology of SVCS and the involvement of the breathing pattern, the opinion of the vascular surgery, hematology-oncology and interventional radiology services was requested; they all met to define a new therapy since the patient had no improvement with the anticoagulant treatment. During this meeting, risk-benefit was assessed and cavography and phlebography were determined as the next step to carry out thrombolysis with alteplase and mechanical thrombectomy + superior vena cava angioplasty + femoral central venous catheter placement; this procedure was performed at 99 days of the child's life (Figure 2).
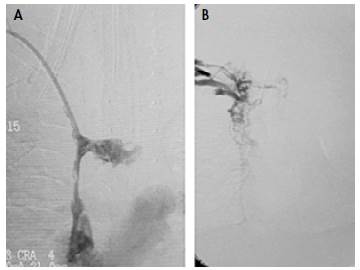
Source: Document obtained during the study.
Figure 2 Fluoroscopic images. A) Vena cava superior thrombosis; B) Superior vena cava patency restored after mechanical thrombectomy before chemical thrombolysis with 0.02 mg/kg/hour alteplase.
The dose of alteplase was adjusted according to the information available for management in adults (0.02 mg/kg/hour). During the procedure, it was found that 70% of the thrombus consisted of purulent material and 30% of hematic material. Partial extraction was achieved, and a sample was sent to pathology; given the findings, broad-spectrum antibiotic was administered with vancomycin and meropenem at maximum doses. During the procedure, the patient presented abundant bleeding, requiring transfusion of blood products: 60mL of packed red blood cells and intravenous fluid resuscitation.
After the procedure, the patient continued receiving low-molecular-weight heparin at 48 UI/kg/hour with thromboplastin time control every 6 hours and dose adjustment according to laboratory values. Although the child did not develop subsequent complications, his condition did not improve, and face and neck edema persisted (a control ultrasound scan showed persistence of left thrombus and right internal jugular vein thrombosis). Therefore, surgery was scheduled at 102 days of age to perform a second mechanical thrombectomy and continue with chemical thrombolysis. After 7 days of continuous infusion of alteplase in the thrombus (in vitro clot lysis effect), a new angiography was performed to evaluate the patient's condition. It was found that 50% of the superior vena cava patency had been restored.
At 107 days of age, a Doppler ultrasound was requested which showed persistence of the left thrombus, patency of the right internal jugular vein partially restored, and patency of the left internal jugular vein and both subclavian veins completely restored. This scan also revealed collateral circulation of the anastomotic complex of the anterior chest wall, which was anastomosed with the azygos veins and the periumbilical epigastrium system; therefore, anticoagulant therapy was continued with unfractionated heparin and dual antiplatelet therapy.
At 128 days of age, the patient presented edema in the right hemiface (hard on palpation and of rapid progression) with collateral circulation to the superficial veins of the neck; imaging studies were performed, finding recurrent thrombosis with absence of flow in the right subclavian vein and the left jugular vein. Given these results, therapy with infused heparin sodium was restarted and this outcome was considered to be the result of antithrombin III deficiency. No blood tests were performed to establish the etiology of his medical condition because the patient had recently received transfusion therapy and it could alter the results. At 146 days of birth and after 56 days of treatment, complete recanalization was achieved.
At 210 days of age, the patient, who was still in nutritional recovery, was discharged from hospital with the indication of treatment with oral anticoagulants and specific care under the guidelines of the outpatient kangaroo program. The hematology service, on an outpatient basis, performed tests that confirmed sticky platelet syndrome (Figures 3 and 4).
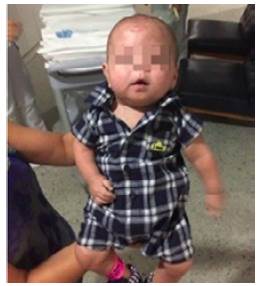
Source: Document obtained during the study.
Figure 3 Patient before being discharged (210 days of life).
Source: Document obtained during the study.
Figure 4 Outpatient pediatric follow-up appointment (224 days of life).
Figure 5 shows the timeline of the patient's he received from birth to discharge at at 210 clinical course, along with the management days of age.
DISCUSSION
Since there is insufficient evidence, the best been established and remains under study. As treatment for children with SVCS has not yet it is a very rare pathology in children and no clear figures have been reported regarding its incidence in this population. Furthermore, the safety and efficacy of drugs administered in newborns is still doubtful. Described treatments include anticoagulant agents such as unfractionated heparin, low-molecular-weight heparin, and thrombolytic therapy with r-TPA (alteplase). Nowadays, thrombectomy is no longer one of the first management options because the size of the blood vessels limits the adequate approach and makes this a procedure of high complexity and morbimortality. 5
The first point to consider regarding medical management is the importance of prevention in patients with central venous catheters. In them, low continuous doses of heparin infusions (0.02-0.03 mg/kg/hour) have been shown to reduce bleeding and to be useful in the prevention of intravascular thrombosis associated with these devices. 6
For anticoagulation therapy, low-molecular-weight heparin is preferred over unfractionated heparin because the former has 100% bioavailability and has 2-4 times longer plasma half-life and dose-independent renal clearance, making its response more predictable. Moreover, unlike unfractionated heparin, which has a higher risk of producing thrombocytopenia and osteoporosis, low-molecular-weight heparin requires less laboratory monitoring and dosage adjustment. 7 On the other hand, unfractionated heparin has the advantage of being rapidly reversible and cheaper, but its pharmacokinetics is unpredictable due to its high ability to bind to proteins and cells; it also has significant side effects such as increased prevalence of bleeding and induced thrombocytopenia, which occurs due to the formation of antibodies against the heparin/ platelet factor complex in at least 1 out of every 100 patients receiving treatment for 5 days or more. 8 If treatment with this drug is decided, a dedicated intravenous catheter must be placed for its administration so that therapy is not interrupted. 9
It should be noted that the management of SVCS in adults is extrapolated to the pediatric population due to the lack of studies, being thrombolytic therapy the treatment of choice. Thus, for example, Gray et al.,10 in a study of 16 patients with central venous catheters who were treated with urokinase (n=11) or streptokinase (n=5), reported that thrombolytic therapy was effective in 73% (n=8) of the patients treated with urokinase and in 20% (n=1) of those treated with streptokinase. Likewise, these authors concluded that fibrinolysis performed through the central venous catheter is more effective since the drugs reach the clot more directly than when the same drug is administered systemically and that the factors predicting successful thrombolysis are 1) using urokinase instead of streptokinase, 2) having a central venous catheter, and 3) symptoms lasting for <5 days.
In general, thrombolytic agents are not recommended for the pediatric population, except in life-threatening conditions due to the high risk of bleeding. In this regard, several authors agree that tissue plasminogen activator is the most widely used fibrinolytic drug. 1,5,11,12 At this point, it is important to mention that fibrinogen levels are the most reliable and fastest indicator of response to thrombolysis, with 1.0 g/L being the accepted lower limit. A platelet count >100 x 10-9 may also indicate a positive response to thrombolytic treatment. 13
Alteplase is a thrombolytic agent often used in cases of central venous catheter thrombosis to achieve local clot lysis with intracatheter administration. However, careful monitoring is required as systemic administration increases the possibility of local blood vessel damage and the formation of new thrombi. 5
As mentioned above, information on the treatment of SVCS is scarce, and available data are obtained from case reports where the dose of alteplase varies. Alvarez et al.14 state that if the gestational age of the newborn is between 24 and 38 weeks, the dose should be 0.1 mg/kg/ hour in continuous infusion, titrating as needed to maintain fibrinogen levels >100 mg/dL; Yang et al.15 established that the dose in newborns should be 0.1-0.15 mg/kg/h; 15 finally, Giglia et al.16 recommend a dose of 0.1-0.6 mg/ kg/hour for infants, children and adolescents.
It should be noted that the most frequent adverse reactions to alteplase are intracranial bleeding (15%), ischemic stroke (6%), ecchymosis (1%), gastrointestinal bleeding (5%) and genitourinary tract bleeding (4%). To a lesser extent (<1%), anaphylaxis, angioedema, arrhythmias, deep vein thrombosis, cerebral herniation, pulmonary edema, and pericardial effusion, among others, may occur. 17
In the present case, anticoagulant management with enoxaparin was initiated without achieving clinical improvement and with thrombus progression to deep vein thrombosis, so pharmacological thrombolysis was performed. Because the symptoms persisted, it was necessary for the interventional radiology service to perform mechanical thrombectomy and angioplasty of the superior vena cava, which restored the patency in 50% of the right internal jugular vein and complete patency of the left internal jugular vein and both subclavian veins. Subsequently, treatment continued with anticoagulants to avoid recurrence of thrombosis at the previously treated site.
With a morbidity of 30% and a mortality of 18%, thromboembolic disorders have a profound impact on public health; they can cause pulmonary embolism (6-8%) and post-thrombotic syndrome (12-19%) and have a significant percentage of recurrence (10-20%). 18 In this scenario, and taking into account that treatment in the pediatric population has not yet been clearly established, it is important to make a risk-benefit comparison to define the best therapy for each patient based on antithrombotic and anticoagulant therapeutic options, especially for the neonatal population. In this sense, it is necessary to stress the importance of conducting therapeutic research in this age group to support the therapies described in medical practice, since the lack of evidence has led to the need to extrapolate valid recommendations in the adult population without adequate consensus.
CONCLUSIONS
Fibrinolytic therapy with alteplase is a viable option to reduce the size of a thrombus. However, it should be borne in mind that the risk of bleeding is markedly increased with this drug, so strict monitoring of patients is required. On the other hand, mechanical thrombectomy is a procedure that, although effective for treating thromboembolic disorders, is difficult to perform on newborns given the diameter of their blood vessels; it is also associated with high rates of thrombotic recurrence.
Strict monitoring of bleeding and taking control lab test allows adjusting anticoagulant doses without having complications such as massive bleeding.