INTRODUCTION
Seizures related to metabolic disorders are common phenomena in many clinical contexts due to hydroelectrolyte alterations, liver, or kidney failure and, more frequently, hypoglycemia. However, clinical manifestations (visual disturbances, convulsions) and imaging findings, in the context of a hyperglycemic crisis, are less frequent and have an unclear pathophysiology.
Seizures are a known complication of hypoglycemia. 1 Conversely, convulsions induced by hyperglycemia, although described in some case reports, do not appear often in clinical practice, as do neurological manifestations of hyperglycemia such as abnormal movements (chorea, athetosis, ballism), headache and visual hallucinations. 2,3 The underlying pathophysiological mechanism of epileptic crises induced by hyperglycemia is not clearly understood, however, they usually have some characteristics in common: they are focal and are associated with an imaging pattern characterized by cortical edema and T2 white matter hypointensity predominating in the posterior and mesial regions. 4 Hyperglycemic crises associated with these complications are always non-ketotic.
The literature describes a few similar cases with characteristic imaging findings for hyperglycemia, which requires considering it as a differential diagnosis in the context of other serious processes such as infections or vascular disorders. The following is the case of a patient with occipital epileptic seizures and imaging findings suggestive of this condition.
CASE REPORT
A 68-year-old-man, right-handed, white, from a middle-income household in Bogotá, unemployed, without relevant medical or family history, presented with polydipsia, polyuria and nocturia for two weeks, accompanied by episodes of time and spatial disorientation, amnestic failures, inattention, and gait abnormality. He consulted the emergency room where hyperglycemia was found at 489 mg/dL; he initially received treatment for the hyperglycemic crisis with hydration and insulin, achieving metabolic control. Subsequently, he was discharged with treatment for type II diabetes mellitus.
The patient attended medical consultation once again a week later because of persistent cognitive impairment, drowsiness, increased confusion, worsening of the gait pattern, and visual complaints. Blood glucose levels were at 400 mg/dLL, so he was admitted to the hospital. During his stay, he presented a focal epileptic crisis manifested with tonic posture of the four limbs and clonic seizure movements of the lower right limb which required the administration of intravenous levetiracetam with consequent seizure improvement.
One day later, he had two seizures with tonic posture of the four limbs accompanied by clonic seizure movements lasting two minutes; benzodiazepines were administered to control the crisis. The patient was somnolent, inattentive, disoriented with right congruous homonymous hemianopia with macular sparing and sensory ataxic gait with right hemihypoesthesia without motor deficit. Initial laboratory tests showed hyperglycemia without other relevant findings.
Brain MRI showed cortical diffusion restriction in occipital, temporal (mesial) and left parietal lobes, with subcortical hypointensity in T2 and F (Figure 1).
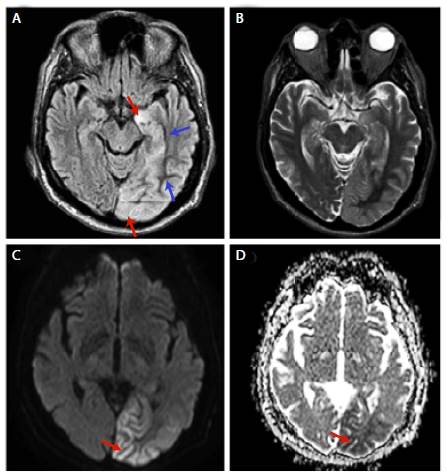
Source: Document obtained during the study.
Figure 1 Brain MRI. A-B: FLAIR (T2-weighted-fluid-attenuated inversion recovery) with subcortical low signal (blue arrow) and cortical high signal (red arrow) in occipital-temporal region extending to the left hippocampal gyrus; C-D: Diffusion restriction in left occipital region (red arrow).
Given this distribution, it was necessary to rule out viral encephalitis vs. paraneoplastic encephalitis; therefore, a lumbar puncture was carried out, finding physical and cytochemical examination of the cerebrospinal fluid within normal limits including negative PCR for type 1 herpes. Study of paraneoplastic syndrome was made, considering limbic encephalitis, obtaining a contrasted thoracoabdominal tomography within normal limits and negative tumor markers.
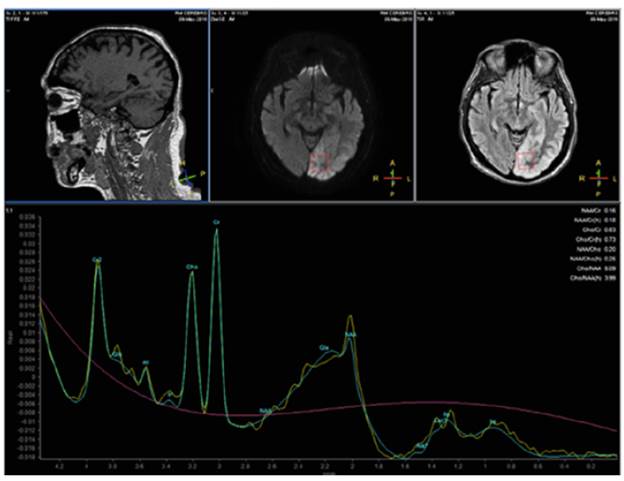
Finally, a single-voxel magnetic resonance spectroscopy (MRS) with echo time (TE) of 35 and 135 ms was performed, which was not specific but allowed ruling out lactate (Figure 2), with an inversion of the choline (Cho)-to-creatine (Cr) ratio in the short echo-time. The patient had a progressive improvement of the clinical symptoms, achieving glycemic control; antiepi-leptic drugs were maintained for three months and reduced progressively until suspension. After a 2-year follow-up, the patient was stable, asymptomatic, and without new seizures. Given his satisfactory clinical evolution, it was not necessary to carry out control neuroimaging.
DISCUSSION
Few cases similar to this have been reported in patients who develop occipital brain focal lesions predominantly after a hyperglycemic crisis, presenting seizures and alteration in the content of consciousness with MRI showing subcortical hypointensity. 5-10
The focal nature of the convulsive crisis in hyperglycemia has been widely described in the literature, and the formation of thrombi in the microcirculation of some brain regions has been proposed. 3 Other authors suggest that malformations of cortical development, such as heterotopia and cortical dysplasia, as well as old vascular or traumatic focal lesions (cerebral infarction or encephalomalacia), are part of the predisposing factors for seizures in patients with hyperglycemia. 11 Another pathophysiological explanation could be the decrease in the expression of Aquaporin-4 (AQP-4) demonstrated in experimental studies with hyperglycemic mice, which predisposes to the development of vasogenic edema and the destruction of the blood-brain barrier. 12 Table 1 shows other similar cases reported in the literature and the main radiological features found.
Table 1 Case reports of occipital seizures or visual symptoms associated with hyperglycemia.
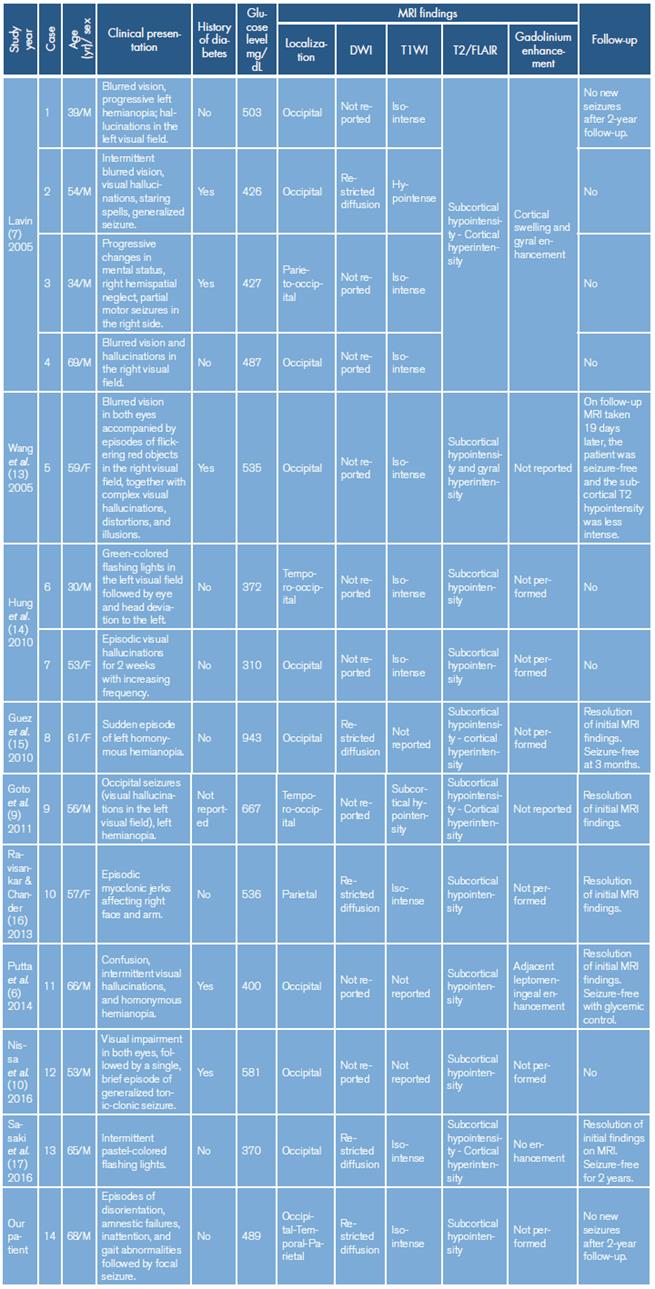
DWI: Diffusion-weighted imaging; T1WI: T1 weighted image; FLAIR/T2: T2-weighted-fluid-attenuated inversion recovery; M: male; F: female.
Source: Own elaboration.
An unsolved question is why seizures are much less frequent in hyperglycemic ketoacidosis. A possible explanation is that ketoacidosis decreases neuronal excitability by increasing GABA levels through the activation of glutamic acid decarboxylase, increasing, in turn, the cellular concentration of glutamic acid and decreasing the GABA "shunt". Another pathophysiological explanation of the convulsions induced by hyperglycemia has to do with potassium ATP channels, whose closure prevents the outflow of potassium that leads to cellular depolarization; so, a hypothesis is that these channels lead to an increase in neuronal excitability in a hyperglycemic environment. 18,19 The importance of mapping the cerebral distribution of these receptors has been mentioned, which would explain the predilection for certain brain areas. 5
Another interesting finding that could lead to additional studies and research is the behavior of spectroscopy in this patient, which showed a relative increase in creatine (Cr) and a decrease in N-acetylaspartate (NAA). The literature has described changes in the NAA/Cr ratio, as experimental biomarkers in animal models, in oxidative disorders of cerebral metabolism. 20
The main differential diagnoses for imaging changes induced by hyperglycemia include post-convulsive edema, autoimmune encephalitis, herpes simplex encephalitis and early and incidental atypical manifestations of primary neoplastic pathology (pattern of gliomatosis cerebri and diffuse astrocytoma). Clinical correlation and imaging follow-up are necessary since the distribution and signal of the MRI alterations are similar in several clinical entities.
To achieve the differential diagnosis between autoimmune encephalitis and herpes virus, clinical and laboratory tests were performed during the hospitalization of this patient, including analysis of cerebrospinal fluid and extension studies. The results clarified the anatomical distribution of the brain injury which, in this case, is not typical for herpes or autoimmune encephalitis due to cortical predilection, unilateral location, poor expansive effect in white matter, or hemorrhagic changes. It should be noted that the anatomical distribution does not correspond to a particular arterial territory, and this, together with spectroscopy, ruled out acute ischemic events.
Finally, the almost null expansive effect of the lesion (particularly in the white matter), would make less probable an infiltrative/neoplastic pattern in the first place. Because our patient fully recovered, and based on the MRI signal characteristics, autoimmune encephalitis was unlikely. Decreased T2 signal changes are better explained as the result of intracellular dehydration of glial and supporting tissue and the probable accumulation of free radicals.
This is a relevant case because this radiological pattern associated with seizures in the context or non-ketotic hyperglycemia is a very rare complication of diabetes and prompt recognition is imperative for an early treatment and seizure control.
CONCLUSIONS
Non-ketotic hyperglycaemic states can manifest with various rare neurological alterations and recognizing them early is of vital importance given their potential reversibility. As in other metabolic disorders, epileptic seizures can be identified in this context because of their focal-type characteristics.
Even though the pathophysiological mechanisms are not clearly elucidated yet, the different neuroimaging techniques promise to establish patterns that allow for an accurate and timely diagnosis. Similarly, differential diagnoses, which are conditions that threaten life, should be part of the approach and routine study of these patients.
A detailed clinical history and laboratory studies, such as cerebrospinal fluid and magnetic resonance, should guide the definitive diagnosis, avoiding unnecessary treatments and interventions that could entail more risks than benefits, and occasionally unnecessary paraclinical studies.
It should be noted that this type of hyperglycemic disorder is poorly understood because of its rare occurrence, which is further affected by underreporting and misdiagnosis.