INTRODUCTION
Myxedema coma is the most extreme expression of hypothyroidism 1. It is now a rare pathology due to universal iodine supplementation, which started in Colombia around the 1950s and has significantly reduced the number of cases of endemic goiter, hypothyroidism, and cognitive retardation, as well as infant mortality in areas with low economic and social development 2.
The pathophysiology of myxedema coma is caused by severe and prolonged thyroid hormone depletion -thyroxine (T4) and triiodothyronine (T3) 3. It is an endocrinological emergency, more frequently observed in women and the elderly and is characterized by a compromised state of consciousness that can lead to coma 3,4. It is associated with exposure to extremely low temperatures and the development of acute conditions such as sepsis secondary to pneumonia, urinary tract infection and cellulitis, which in the case of developed countries are the most frequently reported 1,5. However, other factors have been identified in Latin American countries such as stroke, acute myocardial infarction, polytrauma 3,6,7, or decompensation of chronic diseases such as heart failure 8.
Surprisingly, myxedema coma still occurs despite the widespread availability of tests to assess thyroid function and the regular presentation of changes in these hormones, especially hypothyroidism 5. This condition requires a high degree of diagnostic suspicion, but because of its rarity, it is not commonly regarded as a probable diagnosis in the emergency room, so identification and care are delayed, particularly when it presents with atypical symptoms; this lack of diagnostic suspicion overshadows the prognosis 5,7.
Treatment for myxedema coma is based on intense hormonal replacement with levothyroxine, preferably intravenous (IV), with loading doses up to 400mcg. During the implementation of this treatment, patients with a history of coronary disease or arrhythmias should be treated with special care since it could be harmful, and the necessary support measures should be provided, including physical means to avoid hypothermia, maintenance of hemodynamic stability with vasopressors and inotropic agents (if applicable), and ventilatory support. Other specific measures include starting broad-spectrum antibiotic treatment early if associated infection is suspected, correcting electrolyte imbalance, mainly hyponatremia, and treating other specific triggers discovered 1,3,5,9.
CASE PRESENTATION
A 73-year-old female patient, Hispanic, from the urban area of Manizales (Colombia), with elementary schooling, housewife and with a history of unstratified chronic heart failure, hypothyroidism, high blood pressure and atrial fibrillation without any anticoagulation, and apparent poor adherence to treatment, consulted the emergency department due to a 2-month history of bilateral edema of the lower limbs, predominantly in the evening, non-painful generalized cyanosis, dyspnea on exertion associated with functional class deterioration, orthopnea, paroxysmal nocturnal dyspnea and episodes of lipothymia. The patient reported no chest pain, fever, hemorrhagic manifestations, nor digestive or urinary symptoms.
The patient attended the hospital due to a pronounced physical and functional limitation. On admission, she was medicated with furosemide 40mg every 24 hours, losartan 50mg every 24 hours, metoprolol 25 mg every 12 hours, hydrochlorothiazide 25mg every 24 hours and atorvastatin 40mg every 24 hours. She also reported a long history of toxic exposure to biomass combustion and a surgical history of left inguinal herniorrhaphy and umbilical herniorrhaphy.
Physical examination on admission revealed: respiratory rate: 24 brpm, heart rate: 1 19 bpm, blood pressure: 140/80 mm/Hg, pulse pressure: 60 mm/Hg, mean arterial pressure: 100 mm/Hg, oxygen saturation: 87% on room air and 93% with low-flow oxygen, axillary temperature: 36°C, weight: 64kg, height: 1.47m and body mass index: 29kg/m2. The patient was anxious and had mild respiratory distress, signs of moderate dehydration and cyanosis, anasarca and moon facies, jugular vein distention at 45°, and positive hepatojugular reflux. On inspection and palpation of the neck, there was no evidence of goiter or masses.
The chest was symmetrical, with normal expansion and subcostal retractions, hypoventilated lung fields, and bilateral basal stertor. Heart sounds were arrhythmic, but no cardiac murmurs or carotid bruits were auscultated. The abdomen was globose as a consequence of ascites without collateral circulation, audible peristaltic sounds, and enlarged liver at 4cm from the costal margin, but there was no pain on palpation. Grade 3 edema was found in the lower limbs with fovea, capillary refill for 5 seconds, low-intensity posterior dorsalis pedis artery and tibial artery pulses, and acrocyanosis (Figure 1).
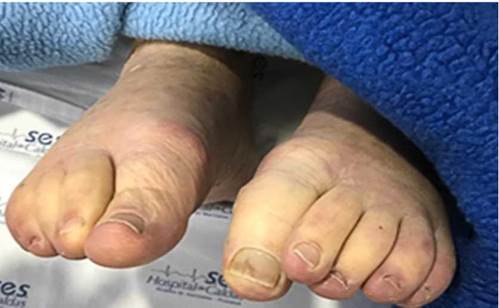
Source: Document obtained during the course of the study.
Figure 1 Lower limb edema and acrocyanosis.
During the neurological examination, the patient was alert, attentive, oriented to place, time and event, and did not present alterations in cranial nerves, strength or sensation. No prior laboratory tests were provided at the time of assessment.
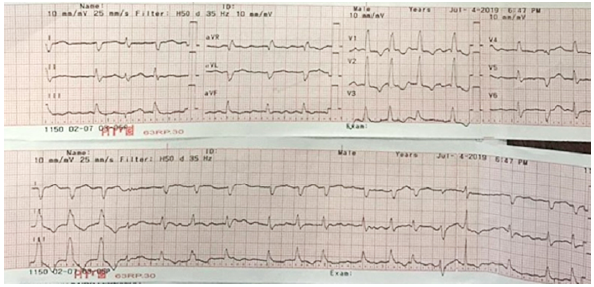
An initial diagnosis of acute chronic heart failure of probable hypertensive etiology was made with the Stevenson B hemodynamic pattern and incipient signs of tissue hypoperfusion. Therefore, treatment was started with supplemental oxygen therapy by low-flow nasal cannula, loop diuretics and prophylaxis for venous thrombosis with low molecular weight heparin (LMWH). Initial examinations included a 12-lead electrocardiogram that showed atrial fibrillation with triplets of wide QRS complex extrasystoles and (Figure 2).
Lab tests results yielded blood arterial gases without oxygenation disorder, compensated respiratory alkalosis, lactate: 2.5 mmol/L, blood count with mild heterogeneous normocytic hypochromic anemia, hemoglobin: 11.2 g/dL, mean corpuscular volume: 81 fL, mean corpuscular hemoglobin: 25.5 pg, red cell distribution width: 17.7% and platelets, leukocytes, sodium, and potassium within normal ranges. Chest x-ray showed an increased cardiothoracic ratio and angiosclerotic changes with calcified plaques and signs of precapillary pulmonary hypertension. Furthermore, mixed involvement of the parenchyma was found with a greater predominance of parahilar alveolar and lower lobe involvement, with a moderate amount of bilateral free fluid predominantly in the left hemithorax (Figure 3).
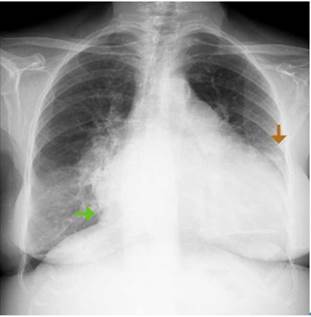
Source: Document obtained during the course of the study.
Figure 3 Chest x-ray. Green arrow: overall cardiomegaly with cardiothoracic ratio of 0.7; Orange arrow: left pleural effusion.
Other tests showed: TSH: 100μIU/mL (normal 0.27-4.2 mIU/mL), free T4: 0.21 ng/dL (normal 0.9-1.7 ng/dL), free T3: 1.61 pmol/L (normal 3.1-6.4 pmol/L), BUN: 18.5mg, creatinine: 1.53 mg/dL, glomerular filtration rate: 33.4 mL/min/1.73 m2 estimated by MDRD, blood glucose: 106 mg/dL, and hs troponin T: 0.031 ng/mL (normal<0.014 ng/mL).
The internal medicine department examined the patient on the next day of admission and diagnosed myxedema coma as a result of acute chronic heart failure with Stevenson C hemodynamic pattern and atrial fibrillation (CHADS2-VAsc score of 4). Therefore, an increase in the LMWH dosage to anticoagulation dose was requested. To rule out acute myocardial infarction, a control troponin test was taken for delta measurement, which was negative (0.034 ng/mL) and corresponded to myocardial injury due to exacerbation of heart failure.
Hormone replacement was initiated with a loading dose of 200mcg of oral levothyroxine and continued at 100mcg/day. Continuous monitoring was also undertaken to determine the need to initiate inotropic support with low dose milrinone (0.375mcg/kg/min) and transfer to the intermediate care unit (IMCU).
A transthoracic echocardiogram showed left ventricular ejection fraction of 57%, moderate right ventricular systolic dysfunction, functional tricuspid regurgitation grade 2/4 with moderate increase in pulmonary systolic pressure, grade 2/4 mitral regurgitation with sclerotic changes, severe dilatation of both atria, and mild pericardial effusion with no hemodynamic effect. A renal ultrasonography showed decreased cortex-medulla ratio as a sign of chronic kidney disease.
Since the patient's condition did not improve, a D-dimer test was requested, finding elevated levels (1 727 ng/mL). Then, to confirm suspicion of pulmonary thromboembolism as an additional cause of decompensation, a chest CT scan with intravenous contrast was performed with previous nephroprotection due to the high risk of nephropathy. Adequate opacity of the vascular structures of the mediastinum was observed, without filling defects or other findings suggestive of acute or chronic thrombotic phenomena. There was an increase in the diameter of the main pulmonary artery of approximately 36mm, global cardiomegaly and scarce fluid in the pericardial sac. Pulmonary parenchymal assessment showed areas of hypoattenuation with mosaic perfusion pattern due to precapillary pulmonary hypertension with moderate amount of free pleural fluid in the right hemithorax and scarce fluid in the contralateral hemithorax with passive atelectasis.
After two days of stay at the IMCU, the patient's condition improved with marked reduction of edema, improvement in dyspnea and gradual recovery of kidney function. However, cyanosis and inotropic support requirement persisted, so hydrocortisone was started at a dose of 50mg every 12 hours. Subsequently, the patient became hypotensive, diaphoretic, desaturated, and bradycardic, so vasopressor support was initiated with norepinephrine. Milrinone was suspended, and an electrocardiogram was taken with no significant new findings.
On the third day at the IMCU, noninvasive mechanical ventilation was initiated because the patient persisted with congestive signs and had respiratory acidosis associated with oxygenation disorder. On the fourth day, vasopressor support was withdrawn, and a follow-up chest x-ray was performed, showing bilateral pleural effusion that was drained the following day without measuring cholesterol, albumin or other markers for the categorization of a probable associated transudate. Improvement in respiratory pattern and acid-base balance in arterial gases was observed, as well as regulation of free T4 on the rise (0.61 ng/dL). 24-hour Holter monitoring was requested.
Due to clinical improvement, the patient was transferred to the general ward on the sixth day of her stay at the IMCU. Holter monitoring showed atrial fibrillation with a maximum ventricular response of 177 beats per minute in wakefulness and a minimum ventricular response of 58 beats per minute in sleep hours, with a mean heart rate of 97 beats per minute.
During hospitalization in the general ward, hydrocortisone was discontinued, and bridging anticoagulation therapy was started with warfarin; the patient persisted with lower limb edema and dyspnea episodes, even with diuretic dose adjustment. Given the slow and torpid evolution, the suspicion of pulmonary thromboembolism persisted, so pulmonary perfusion and ventilation (V/Q) scans were requested, which showed multiple segmental perfusion defects that involved the posterior basal and anterior basal faces in the right lower lobe and the lateral surface in the middle lobe. There was also an involvement of the apical segment of the left upper lobe and segments of the lingula. The findings were highly suggestive of bilateral multiple pulmonary thromboembolism (Figures 4 and 5).
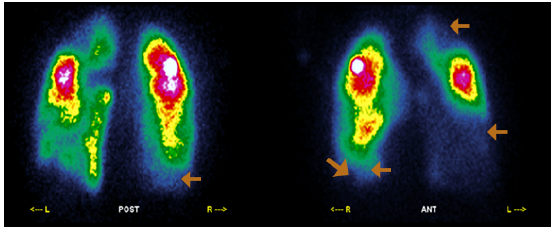
Orange arrows: perfusion deficits in the basal anterior, lateral and posterior segments of the right lower lobe, in the apical segment of the left upper lobe, and in segments of the lingula. Source: Document obtained during the course of the study.
Figure 4 Perfusion scintigraphy.
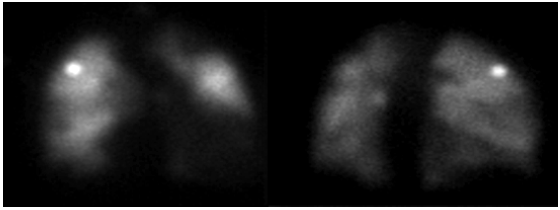
Note: The study could not be performed due to the patient's inability to properly inhale the radiopharmaceutical agent. Source: Document obtained during the course of the study.
Figure 5 Ventilation lung scan.
The anticoagulation therapy allowed achieving the target international normalized ratio by increasing the coumarin dose and improving the free T4 levels (0.8 ng/dL), although without achieving a normal range (lower limit 0.9 ng/dL). A BNP of 19.791 pg/mL was also obtained, which will be useful as a reference in future outpatient monitoring. The patient was discharged 12 days after her stay in the general ward and after 20 days of her admission, when she had an evident improvement in her clinical condition. However, due to her comorbidities and persistent hypoxemia, home oxygen was ordered.
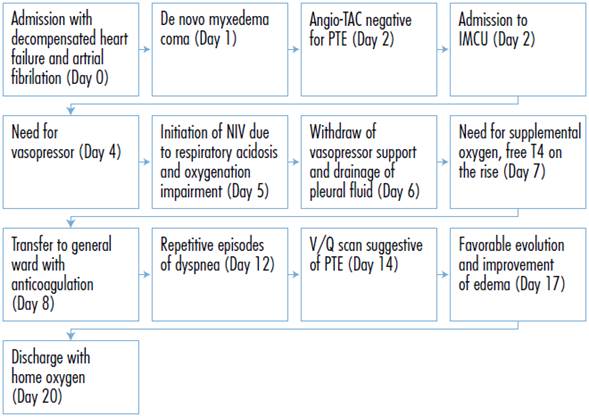
Figure 6 presents the case timeline. No follow-up diagnostic tests are available.
DISCUSSION
Thyroid hormones (T4 and T3) play a key role in human health, mainly in cell growth, differentiation, and metabolism 10. Therefore, it is not surprising that its deficiency is one of the most common endocrine disorders in clinical practice 5,10.
Myxedema coma is the most extreme expression of thyroid hormone deficiency, and its presentation is rare nowadays. This clinical condition, which, contrary to what its name indicates, does not involve the alteration of the state of consciousness in most cases, is characterized by being more usual in the female sex and causing alterations in the thermoregulation and hemodynamic status (the latter is a major clinical finding accompanied by hypotension and other signs of hypoperfusion). It is also associated with glomerular and tubular alterations that reduce the homeostatic capacity to respond to triggering disorders such as pulmonary thromboembolism and heart failure, which should be recognized early to establish specific treatment 3,8.
Hyponatremia is one of the most frequent electrolytic alterations associated with myxedema coma 11; however, in the present case, it did not occur. On the other hand, the most common electrocardiographic alteration in hypothyroidism is bradycardia, although other arrhythmias that are detected in electrocardiography are also described 3,12, as in the case of the reported patient. This could be explained, among other things, by myxedema accumulation due to mucopolysaccharides, particularly atrial fibrillation possibly caused by dilatation of the right atrium associated with the chronic increase of the afterload.
Sequential electrocardiograms should be performed in the emergency department when ischemia is suspected 12. In the reported patient, there was no clinical or electrocardiographic suspicion since no waves of injury, ischemia or necrosis were shown on the electrocardiogram upon admission.
An assessment scale developed in Washington D.C., USA, allows for an objective review of the clinical findings reported for myxedema coma and, as a result, a prompt and accurate diagnosis 13.
The specific treatment of the myxedema coma is based on hormone replacement therapy, preferably by IV route, as well as on the treatment of precipitating entities, general support measures and the prevention of the multiple complications, among which glucocorticoid supplementation stands out. In this way, it is possible to prevent adrenal insufficiency, as indicated in the American Thyroid Association's hypothyroidism management guidelines 9. The ideal place for the treatment of this complication is the intensive care unit (ICU) 13 since patients may need vasopressor, inotropic, and ventilatory support. Response is assessed based on clinical improvement (hemodynamic, neurological, and metabolic), which in turn improves cardiac and pulmonary function 1,9.
Poor prognostic factors in myxedema coma include hypotension, hypothermia, sepsis, altered consciousness (low Glasgow score), and a high APACHE II score, the latter two being the main factors related to mortality 14.
Some strengths of this case report include a detailed description of clinical manifestations, laboratory findings and diagnostic imaging, as well as the patient's evolution and treatment. This is valuable in order not to lose sight of the fact that this type of severe disease is still present today. It also helps to illustrate that, despite the negative result of chest angiotomography, the clinical hypothesis of associated pulmonary thromboembolism persisted, leading to the use of V/Q scintigraphy as a secondary diagnostic method that made it possible to confirm the clinical suspicion. Furthermore, the use of clinimetric instruments allowed for a more objective definition of the condition's onset and severity. One of the study's drawbacks is that there was no adequate outpatient follow-up of the signs and symptoms.
CONCLUSIONS
Myxedema coma is a condition that most often occurs in women, the elderly, and patients with comorbidities. Although in most cases it is not associated with a true coma, it can cause high levels of morbidity and mortality because it involves serious systemic manifestations.
Arterial hypertension, heart failure, pulmonary thromboembolism and atrial fibrillation are conditions associated with the presence of myxedema coma.
Timely diagnosis and early implementation of supportive and specific treatment, especially with aggressive thyroid hormone replacement therapy and intensive care unit monitoring, help to improve the prognosis of patients with this complication.