INTRODUCTION
Tubo-ovarian abscess (TOA) is a complication of pelvic inflammatory disease (PID), which consists of the formation of a purulent collection and distortion of the normal structure of the fallopian tubes and ovary 1. TOA may be accompanied by disabling complications such as pelvic pain, ectopic pregnancy, and rupture of the abscess or intestinal obstruction 2. This type of abscess accounts for 1-2% of admissions to gynecology services and usually occurs in women of reproductive age after exposure to sexually transmitted infections 3, although it has also been observed without preceding sexual activity.
Risk factors for developing a TOA include demographic variables such as low socioeconomic status, risky sexual behavior (multiple sexual partners, previous episodes of PID and early onset of unprotected sex), and recent application of intrauterine devices 3. Age is also a relevant risk factor since it has been found that the younger the age, the higher the risk of PID.
Other factors that may contribute to the development of this phenomenon are cervicovaginal microbiota, cervical ectropion with a larger transformation zone that favors the exposure of columnar epithelium to sexually transmitted infections, and the higher frequency of risky sexual behavior in young patients 4.
Only 1.7% of TOA occurs in postmenopausal women 5. It should be noted that this complication is associated both with benign gynecological conditions (stage III and IV endometriosis, endometrioma, endometrial polyp, uterine leiomyoma) 6,7 and with malignant gynecological and non-gynecological diseases (endometrial adenocarcinoma, malignant epithelial and non-epithelial ovarian tumors, squamous cell carcinoma of the cervix and adenocarcinoma of the colon) 1,8-10.
CASE PRESENTATION
A 72-year-old female patient from Bogotá, Colombia, Hispanic, housewife and affiliated to the public health scheme, attended the gynecology service of a quaternary care university hospital due to abdominal distention associated with intermittent urinary retention, urinary irritative symptoms, and 3 days unquantified fever.
The patient had consulted another health care institution two weeks earlier for similar symptoms and was diagnosed with upper urinary tract infection by Escherichia coli resistant to quinolones and ampicillin. She received hospital treatment with a third-generation cephalosporin for 10 days.
Relevant medical history included high blood pressure and stage 3A chronic kidney disease. She had also undergone cystopexy with mesh reinforcement 10 years ago and had 5 pregnancies, 4 deliveries and 1 abortion. At the time of consultation, she had no active sex life and had no postmenopausal bleeding. No data were obtained from the last cytology.
On admission examination, the patient was found with normal vital signs, distended abdomen with diffuse pain, no signs of peritoneal irritation, and positive bilateral fist percussion. No mass was palpated in the abdomen. The gynecological examination established that the external genitalia were atrophic. Bimanual palpation revealed elastic vagina with normal temperature and short, atrophic, and closed cervix displaced anteriorly by a painful, firm, fixed mass of about 12cm in diameter that occupied the bottom of the recto-uterine pouch and distended the posterior fornix. This mass prevented the individualization of the uterus and its adnexa and gave the clinical impression of a pelvic abscess, without being able to rule out an adnexal tumor. The results of the admission lab tests are shown in Table 1.
Table 1 Lab test results
| Date | Laboratory | Results |
|---|---|---|
| 5/12/2019 (admission) | Hemoglobin | 14 g/dL |
| Leukocytes | 21.880/mm3 | |
| Neutrophils | 19.290/mm3 | |
| Platelets | 425.000/mm3 | |
| Creatinine | 0.6 mg/dL | |
| BUN | 12.8 mg/dL | |
| Electrolytes | Normal | |
| Arterial gases | Normal | |
| 7/12/2019 | Lactate dehydrogenase | 223 U/L |
| 7/12/2019 | BhCG: 0.14 mlU/mL | 0.14 mlU/mL |
| 10/12/2019 | CA-125 | 222 U/L |
| 10/12/2019 | Carcino-embryonic antigen | 1.40 ng/mL |
| 10/12/2017 | Alpha-fetoprotein | 1 UI/mL |
Source: Own elaboration.
The initial patient's diagnosis was a recurrent complicated urinary tract infection and, given the risk of a multidrug-resistant bacteria, antibiotic treatment was initiated with meropenem (1g every 8 hours intravenously (IV)). A transabdominal and transvaginal ultrasound was subsequently performed, showing a solid-cystic lesion of 115x62x139mm located in the recto-uterine pouch and with low-resistance Doppler flow at the center of the lesion. The mass pushed the uterus forward (Figure 1).
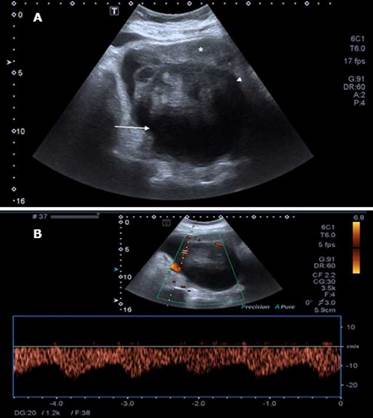
Source: Document obtained during the course of the study.
Figure 1 Transabdominal ultrasound obtained using Toshiba Xario 100 with 6mHz convex transducer. A) Uterus (*) displaced by a solid (arrowhead) and cystic (arrow) lesion; B) Doppler of the lesion with low-resistance flow in the solid component.
A contrast-enhanced computed tomography (CT) of the abdomen and pelvis showed that the mass had the appearance of a pelvic abscess with liquid density lesions with multiple septa in both adnexa. These lesions were accompanied by edema of the surrounding fat, reactive-looking lymph nodes in retroperitoneum, and scarce free fluid in the cavity. It should be noted that the solid component of the mass was not evident with this diagnostic method (Figure 2).
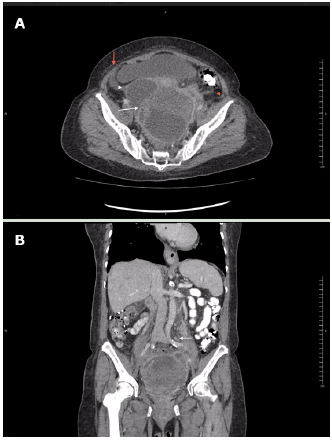
Source: Document obtained during the course of the study.
Figure 2 Contrast-enhanced CT scan obtained during arterial phase using an 80-slice multidetector system by Toshiba. A) axial plane: left ovarian lesion with multiple septa, hypodense center, marginal enhancement (white arrow), adjacent fat stranding (red arrowhead) and scarce free fluid (red arrow), and cystic-looking lesion in right adnexa (white arrowhead); B) coronal plane: multiple, retroperitoneal lymph nodes (white arrow).
The patient was evaluated by the gynecologic oncology service, which considered the possibility of ovarian carcinoma with a low probability of abscess. Contrasted magnetic resonance imaging (MRI) was requested, finding right adnexal mass compatible with simple cyst and left adnexal mass with solid and cystic component of 152x120x-103mm; the solid zone had low signal on T2, without enhancement or restriction, and the cystic component showed significant restricted diffusion and low apparent diffusion coefficient (ADC). This technique also showed that the mass pushed the uterus, bladder, and rectum, and confirmed the presence of scarce fluid in the cavity (Figure 3).
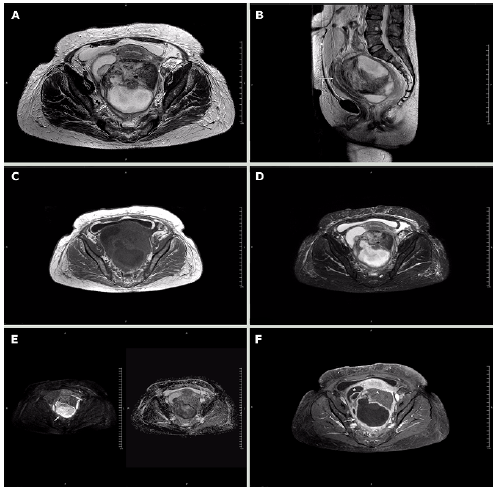
Note: The cystic component is marked in B) with the arrow and shows restricted diffusion in G); the solid component does not show restricted diffusion (G-arrowhead) or enhancement (H-arrowhead). The displacement of the uterus (arrow) and bladder (arrowhead) is evident in C). The asterisk in F) marks the location of the right ovarian cyst. Source: Document obtained during the course of the study.
Figure 3 Magnetic resonance imaging obtained using a 1.5T Philips MRI machine. Axial T2-weighted slices. A) axial plane; B) sagittal plane; C) axial T1; D) axial STIR sequence; E) B500 and axial ADC map; F) axial T1 with fat suppression and contrast.
Given the findings, a complex adnexal mass M3 was considered. The risk of malignancy index was IRM-1: 1998 and IOTA - ADNEX model: 8 (84% malignancy) (Table 1).
Upper digestive tract endoscopy was performed, finding grade B erosive esophagitis and chronic gastritis, and a colonoscopy showed grade II internal hemorrhoids and diverticulosis; a colon biopsy was taken. The patient was again assessed by gynecologic oncology, which considered the possibility of ovarian carcinoma with a low probability of abscess.
A CT-guided biopsy was performed for histological study, staging and evaluation of surgical benefit, finding abundant purulent foul-smelling material. In view of the diagnostic doubt of malignant tumor versus TOA, an exploratory laparotomy was performed using a midline laparotomy infraumbilical incision.
Laparotomy showed a double-lobed retrouterine pelvic mass of 10x7x5cm and solid-cystic appearance. It was attached to the pelvic walls, the posterior side of the uterus, the pouch of Douglas, and the anterior surface of the sigmoid rectum. One of the locules of the mass had a smooth, purplish, renitent surface with liquid content. The mass had a 6cm light brown solid component in one of its poles, thick walls, and it was firmly adhered to neighboring structures. It was also full of foul-smelling purulent material (Figure 4).
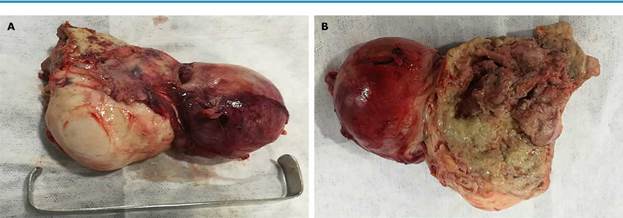
Source: Own elaboration.
Figure 4 Surgical specimen of pelvic mass. A) ovarian fibroma (solid) on the left and abscess (cystic component) on the right; B) thickened and fibrinopurulent walls of the abscess on the right side of the solid component.
Laparotomy also established that the Cook 8 Fr multipurpose drainage catheter that had been placed by interventional radiology was well located in the abscess and that the fallopian tubes were edematous and involved in the inflammatory process. In addition, serous fluid was found on the subdiaphragmatic surface.
Due to the patient's condition, purulent material culture, bilateral salpingo-oophorectomy, multipurpose catheter removal, drainage, and abdominopelvic cavity washing were performed. Jackson-Pratt drains were left in the pouch of Douglas and the abdominal wall was sutured in planes. On the fourth postoperative day, along with the infectious diseases service, the antibiotic treatment was switched to piperacillin-tazobactam (4.5g every 8 hours IV) after obtaining negative urine and blood cultures at 72 hours.
Pathology report revealed benign stromal ovarian fibroid tumor with areas of necrosis, neutrophil infiltrate, congestion, and edema, corresponding to a TOA. Subdiaphragmatic fluid samples with reactive mesothelial hyperplasia were negative for malignancy, as were colon biopsies obtained during colonoscopy. Enterococcus raffinosus of the usual pattern was isolated in the purulent fluid of the pelvic collection.
Following the surgical procedure, acute kidney injury KDIGO 3 was observed, which was associated with drug-induced nephrotoxicity (dipyrone and metoclopramide) and resolved upon discontinuation. The patient progressed satisfactorily, was discharged 5 days after surgery and completed 14 days of parenteral antibiotic treatment without adverse reactions. The patient was asymptomatic during follow-ups at one week and one month after surgery.
DISCUSSION
TOA is a condition rarely observed after menopause. Gockley et al.11, in a retrospective study with 61 postmenopausal women, found a wide range in the age of presentation of this disease -from 50 to 87 years-, which coincides with other retrospective studies and case reports 5,6,9,10.
TOA is usually polymicrobial with aerobic and anaerobic bacteria. Enterococcus fecalis, Escherichia coli, Bacteroides fragilis, Pep-tostreptococcus magnus, Steptococcus sp, Pseudomonas aeruginosa and Clostridium perfringens can be isolated 6.
Most patients with TOA present with abdominal pain as the first symptom (84%), followed by fever (34%), nausea or vomiting (28%), or vaginal bleeding (45%). This condition is also accompanied by comorbidities such as diverticulitis (34%), high blood pressure (25%), and diabetes (10%) 6,9.
37% of women with TOA have history of a recent pelvic surgery. On physical examination, a palpable mass can be found in only 55% of cases; signs of peritoneal irritation are not common 6. In laboratory studies, the levels of leukocytes (mean 13.700/mm3) and CA-125 are usually high, the latter in 77% of patients (mean 101 U/mL), as was the case of our patient. The size of the abscess varies with a mean of 6.0cm (range 1-15cm), and there seems to be no preference for laterality, and at least 10% of cases are bilateral 11.
The patient in the present case was admitted with symptoms similar to those reported in the literature and irritative urinary symptoms that, together with the recent history of urinary tract infection, initially led to suspect the recurrence or persistence of a urinary tract infection. However, based on her clinical evolution and the findings of the abdominal examination, imaging studies were requested; those results, together with elevated levels of CA-125, led to suspect ovarian carcinoma, pelvic abscess-TOA, and abscessed tumor.
The main purpose of the evaluation of an adnexal mass is to rule out malignancy, but since there are no non-invasive techniques to diagnose ovarian cancer, surgical exploration is required, which in many women results in the extraction of benign masses 12.
The treatment of TOA in post-menopause consists of the rapid initiation of broad-spectrum antibiotics, parenteral clindamycin 600mg IV every 6 hours plus gentamicin 3-5 mg/kg IV every 24 hours, cefotetan 2g IV every 12 hours plus doxycycline 100mg IV every 12 hours, or ampicillin/sulbactam 3g IV every 6 hours plus doxycycline 100mg IV every 12 hours 13. IV antibiotic therapy may be switched to oral therapy after 24 hours of clinical improvement, and the recommendation is to complete 14 days with doxycycline. If TOA is preceded or associated with a recent gynecological procedure, extended coverage with metronidazole or clindamycin should be added for anaerobes 14,15.
It should be noted that the decision to combine antibiotic therapy with surgical drainage depends on the patient's clinical condition and the size of the abscess 14. When the patient has clinical deterioration, signs of sepsis, or suspected abscess rupture, surgical management should be performed. If the patient is stable, the decision to perform surgery will be made taking into account the size of the abscess (even though there is no consensus, some authors have proposed that surgery should be performed when the abscess is between 5 and 8cm in diameter) 14-16.
Some authors have found that women in post-menopause are more likely to develop malignant tumors, both gynecological and non-gynecological, compared to pre-menopause women 12,17. For example, Gockley et al.11 found in their study that 13.1% of patients with TOA had cancer; four of them had endometrial adenocarcinoma, one had mucinous borderline tumor, two had uterine malignancies, and one had colon adenocarcinoma. Lipscomb & Ling 6 also showed a higher incidence (30%) of malignancy, including endometrial adenocar-cinoma and serous malignant ovarian tumor.
Given the high frequency of co-existing cancer with TOA, it has been proposed that surgical management in post-menopause should be individualized and dependent on risk factors for malignancy and differential diagnoses, because in this age group, intraoperative and post-surgical complications are more frequent and include sepsis, infection of the operative site, intestinal injury, and rectovaginal fistulas 11,14.
On the other hand, some case reports and retrospective cohorts have found coexistence between TOA and benign lesions in postmenopausal women, including endometrial polyps (10%), uterine fibroids (3%), and benign serous cystadenomas (2%), usually diagnosed incidentally 6,7,10,11,17. In the present case, the diagnosis was made based on a pelvic mass approach, in which, due to the age group, imaging findings and tumor markers led to suspect a malignant ovarian tumor. However, the CT guided biopsy allowed finding purulent material, so surgical management was decided, revealing the co-existence of TAO with ovarian fibroma.
Ovarian fibroma is a benign ovarian sex cord-stromal tumor, which is made up of collagen-producing fibroblasts, accounting for 4% of ovarian neoplasms. It is the most common pure ovarian stromal tumor 18 and is most common in adolescents and young women. At least 1% of these tumors may be associated with ascites and hydrothorax (Meigs syndrome), and 11.3%, with increased CA-125 levels (range of 36-1848 U/mL), which are higher in large tumors (>10cm in diameter).
Due to its clinical, imaging, and serological characteristics, ovarian fibroma is often mistaken for malignant epithelial tumors in postmenopausal women, so these patients are usually taken to surgical procedures (oophorectomy or unilateral salpingo-oophorectomy). In general, the prognosis of this type of fibroma is good and the recurrence rate is very low 19,20.
The literature review conducted for this report yielded no record of concomitant TAO and ovarian fibroma in postmenopausal patients; however, as mentioned above, some other benign ovarian tumors may co-exist with TOA.
In the present case, TOA explains the leukocytosis with neutrophilia presented by the patient, as well as the unquantified fever, since no persistent or recurrent urinary tract infection was demonstrated. CA-125 can be elevated in malignant epithelial ovarian tumors, but also in physiological conditions such as menstruation and pregnancy and in benign processes such as endometriosis and inflammatory diseases of the peritoneum 21-23, such as the patient in the present case.
Several malignancy indexes have been developed using biomarkers to identify which patients with adnexal masses should be referred to gynecologic oncology, including OVA1 and ROMA 12,24 and MIA2G (overa ®); the latter uses the following biomarker results: apolipo-protein A-1, CA-125, human epididymis protein 4, follicle-stimulating hormone, and transferrin. Fredericks et al.25 found that the combination of a positive MIA2G test result with an ultrasound had greater sensitivity (93.5%) and specificity (85%) than either of the two individual tests.
From the point of view of diagnostic imaging, in general, complex ovarian or pelvic masses in postmenopausal patients should make the clinician consider malignancies, especially when accompanied by one or more positive tumor markers; however, some characteristics may help discriminate between benign and malignant lesions. In this sense, radiologists take clinical and laboratory characteristics as pretest odds to calculate the final risk of malignancy.
According to the literature, there are more than 80 predictors of ovarian malignancy, among which ADNEX stands out. It is based on the imaging criteria described by the IOTA group (International Ovarian Tumor Analysis) and has an area under the ROC curve of 0.943 (95%CI: 0.934-0.952) to differentiate between benign and malignant lesions 26-29. Additionally, 0-RADS is an ovarian-adnexal imaging-reporting-data system that provides a risk stratification designed to make consistent interpretations, assign a risk of malignancy, and give a treatment recommendation 30.
According to the IOTA group consensus and the studies that support it, malignancy predictors in ultrasounds include the presence of solid and cystic components, papillary projections, multiple septa, thick septa, and Doppler flow in the solid component; the total size of the lesion and the size of the solid component are also considered 22,23.
Characterization of indeterminate masses is not easy, but complementary imaging studies perform as follows:
Doppler: sensitivity 84% (95%CI: 81-97) and specificity 82% (95%CI: 79-85)
CT scan: sensitivity 81% (95%CI: 73-85%) and specificity 87% (95%CI: 81-94)
Simple MRI: sensitivity 76% (95%CI: 70-82) and specificity 97% (95%CI: 95-98)
Contrast-enhanced MRI: sensitivity 81% (95%CI: 77-84) and specificity 98% (95%CI: 97-99)
Finally, it should be noted that currently one of the best predictors of ovarian malignancy, along with contrast, is the ADC value. A low value in a solid component suggests a malignant lesion, while a low value in a liquid component suggests an abscess. In the present case, a low ADC was found in the liquid component, which is in favor of abscess, and a high ADC in the solid component, suggesting a benign tumor lesion 32.
CONCLUSIONS
The reported case illustrates how, sometimes, a possible diagnosis of ovarian cancer carcinomatosis may actually be a benign condition, such as TOA, which responds well to medical-surgical treatment. Diagnostic imaging and tumor markers are of great help in differentiating a malignant ovarian condition from a benign process.














