INTRODUCTION
Methotrexate (MTX) is an essential drug for the treatment of certain oncological diseases since it increases survival in cancer patients. While it may have cytotoxic effects, they may be partially antagonized by the administration of folic acid, allowing for its usage at high doses. Even though manifestations of MTX toxicity are rare, they occur in 2-12% of treatment cycles and lead to clinical condition scenarios with high morbidity and mortality, such as hepatotoxicity, gastrointestinal mucositis, bone marrow suppression, neurotoxicity, and nephrotoxicity 1,2.
Although several population pharmacokinetic models of high-dose MTX for children have been developed to predict adverse events during chemotherapy, they are complicated and of little use in clinical practice, so serum drug concentration monitoring remains the gold standard for identifying patients at high risk of developing toxicity. It is important to note that there are specific drug concentration values depending on the time of administration 2.
As has been demonstrated in various case series, the management of MTX toxicity, in addition to controlling drug levels to achieve therapeutic goals, aims to a) promote renal perfusion to facilitate clearance of the drug, b) look for effective urine alkalinization levels that avoid precipitation, and c) block unfavorable effects through competitive inhibition or enzymatic cleavage 3,4.
The following is the case of an oncologic patient with preserved renal function who developed nephrotoxicity secondary to the use of MTX. A description of the diagnosis and treatment options is provided below.
CASE PRESENTATION
This is the case of a 71-year-old man from Boyacá, Colombia, with high blood pressure, hearing loss in the right ear, and activated stage IV large B cell non-Hodgkin's lymphoma (central nervous system involvement due to bone marrow mass at the right petroclival junction) diagnosed in February 2020.
The patient, who was admitted in July 2020 to a quaternary care hospital for the administration of a second cycle of scheduled polychemotherapy, had already received a first cycle of this treatment in April 2020, which included rituximab, methotrexate, cytarabine, prednisolone, and filgrastim 5.
During the clinical assessment on admission for his second chemotherapy cycle, the patient presented the following vital signs: blood pressure of 130/80 mmHg, heart rate of 80 bpm, respiratory rate of 16 rpm, temperature of 36.5°C, oxygen saturation of 95% with FiO2 0.21, weight of 64kg, height of 163cm, body mass index of 24 kg/m2, and body surface area 1.68m2 using the DuBois formula. According to the Eastern Cooperative Oncology Group (ECOG) Performance Status scale, the patient rated as PS 1, indicating functional independence. As a relevant finding, the man was found to have right external strabismus as a sign of involvement of the sixth cranial nerve.
It should be noted that the second chemotherapy cycle was initiated in July 2020, one month later than initially scheduled, because the patient presented with left ear pinna perichondritis 15 days after the first chemotherapy cycle started. The infection was treated with antibiotic therapy for 21 days and he had an adequate response.
On the third day after starting the second chemotherapy cycle, the patient developed stage 3 acute renal failure (ARF) according to the KDIGO guidelines due to an increase in creatinine levels: a change from 1.05 mg/dL on admission to 3.25 mg/dL at 48 hours (Table 1). This chemotherapy treatment included the administration of 3 500 mg/m2 of MTX intravenously.
Table 1 Evolution of laboratory tests during hospital stay.
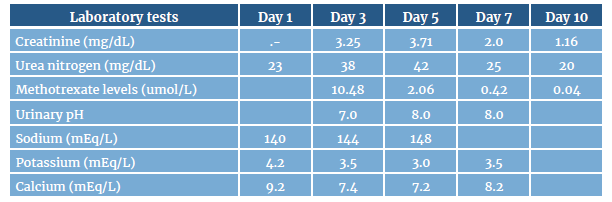
Note: the following are the methotrexate levels that increase the risk of adverse effects: >10 umol/L at 24 hours, >1 umol/L at 48 hours, and >0.1 umol/L at 72 hours.
Source: Own elaboration.
In the partial urine samples, there were no striking findings or crystals typical of drug precipitation. Other causes of ARF were ruled out on the third day of treatment due to the transient relationship between MTX administration and renal function impairment, as well as the absence of intercurrent infectious, hemodynamic, or toxic phenomena and the adequate response to the treatment established, as creatinine and urea nitrogen levels improved from day 7 with the decrease of MTX in serum levels after suspension and the application of the treatment pillars for MTX toxicity (Table 1).
According to Naranjo et al.6, all of the above is classified as a probable toxicity event associated with MTX because the patient obtained a score of 6 points (5-8 points: probable) after applying the adverse drug reaction algorithm published by them. No toxicity was observed in other organs.
Intravenous crystalloid fluids were optimized on the third day at 3-4 liters/day after starting the second chemotherapy cycle and given the patient's condition. Similarly, 50mg of calcium folinate were administered orally every 4 hours, as well as intravenous sodium bicarbonate at a rate of 40 mEq per liter of intravenous crystalloid fluids, with the goal of achieving a urinary pH >7. It should be noted that during the administration of this infusion, there were changes in the levels of water electrolytes, such as hypernatremia and hypocalcemia, which were expected during the treatment; however, those changes did not have an impact on the clinical condition of the patient but were followed up until they were normalized.
The patient's progress was satisfactory, achieving a progressive decrease in creatinine and urea nitrogen levels and reaching baseline levels at 10 days. He also had adequate serum MTX clearance. Considering his favorable progress, it was not necessary to initiate renal replacement therapy.
Taking into account the patient's situation, the hematology oncology service decided to suspend the chemotherapy protocol after one month due to the high risk of renal complications and indicated treatment with external beam radiation therapy using 3D-CRT planning technique with computerized simulation at doses of 300-3000 cGy in PTV-CTV (right petroclival mass plus margin).
DISCUSSION
According to Perazella & Moeckel 7, the overall incidence rate of ARF is approximately 1.8% (range: 0%-12%) and, in general, this type of injury is reversible.
The theory that more consistently explains the mechanism by which MTX-induced nephrotoxicity occurs indicates that it is mediated by the precipitation of this substance and its metabolites in the renal tubules, as well as by direct toxicity in these structures 4. In cases of MTX nephrotoxicity, urinalysis may often show tubular epithelial cells, granular casts and, to a lesser extent, drug crystals if urine is acidic 7.
Regarding factors that increase the risk of nephrotoxicity, Amitai et al.8, in a retrospective single-center cohort study that included patients treated with high-dose MTX in the hematology ward of an Israeli hematology institute between January 2012 and February 2017 (n=160), found that lactate dehydrogenase levels >380 U/L and albumin levels <3.6 g/dL were the factors associated with the development of ARF with the greatest statistical significance.
High doses of MTX (>500 mg/m2 in children and >1000 mg/m2 in adults) used in various oncological diseases, including lymphomas and sarcomas, predictably increase the development of nephropathy, in part because chemotherapy doses used in solid tumors are higher than those used in hematologic malignancies 9.
On the other hand, SKärby et al.10, in a study aimed at determining the relationship between MTX clearance time and various aspects of renal function, established that an increase in serum creatinine by more than 50% is a better predictor of delayed clearance than serum MTX at the end of the infusion, especially if information on previous creatinine measurements is used to reduce the impact of an occasionally low serum creatinine value prior to the start of the infusion. Likewise, according to Iqbal et al.11 in their case report, significant genetic variants affecting MTX disposition and effects have been described in pediatric patients with acute lymphoblastic leukemia, with the strongest variant residing in the reasonable candidate gene SLCO1B1, which is associated with the clearance of this medication.
In addition, it is critical to keep in mind that cancer patients generally receive adjunctive therapies, either because of the oncological disease or because of their underlying comorbidities, and thus some drugs may impair MTX clearance. These drugs include non-steroidal anti-inflammatory drugs, penicillin and its derivatives, salicylates, gemfibrozil, trimethoprim with sulfamethoxazole, amphotericin B, aminoglycosides, proton pump inhibitors, levetiracetam, among others 12.
MTX nephrotoxicity perpetuates ARF caused by this drug; as a result, this failure reduces drug clearance, prolongs toxic serum values over time, and, thus, generates adverse effects such as myelosuppression, mucositis, hepatotoxicity, conjunctival involvement, pulmonary toxicity, among others 12.
Regarding the treatment of MTX nephrotoxicity, it is important to point out that definitive discontinuation of the drug is not completely necessary. In fact, there are several pillars for the prevention and management of this condition, which include urine alkalinization, maintenance of urine output by means of intravenous crystalloid fluids, monitoring of serum creatinine and MTX levels, as well as pharmacological rescue with leucovorin (5-formyltetrahydrofolate) 4.
With regard to urinary alkalinization, a therapeutic possibility to consider is the use of intravenous sodium bicarbonate, which must be adjusted to maintain urine pH levels >7.0 and can be added to the perfusion in punctual boluses; this alkalinization can be verified later in the urinalysis 12,13. There is evidence that acetazolamide can be used to treat metabolic alkalosis in patients who do not have adequate urinary alkalinization 14.
Surprisingly, enteral alkalinization protocols have been studied as well. One of them is the one proposed by Kramer et al.15, which supports the ability to conserve intravenous sodium bicarbonate by using an enteral-based urine alkalization regimen of high-dose MTX (no differences in outcomes or toxicity). However, individuals who received enteral-based urine alkalinization experienced more frequent diarrhea, lower serum bicarbonate levels, and urine pH readings that were slightly below target.
Concerning the maintenance of urinary output, it is expected that adequate administration of intravenous crystalloid fluids prior to and up to two hours after the MTX infusion will facilitate its clearance since 90% of the drug is eliminated by this route 4,8. It is worth noting that studies such as Kinoshita et al.16 associate high sodium concentrations in MTX infusion (70 mEq/L versus 100 mEq/L) with better drug clearance when serum concentrations are monitored during chemotherapy.
About the use of medicinal products, Widemann et al.17 state that pharmacological interventions to treat MTX nephrotoxicity include high-dose leucovorin, glucarpidase, or thymidine. Leucovorin is a folate derivative (5-formyltetrahy-drofolate) that enters the cell in the same way that MTX does and is extensively converted to its active metabolite 5-methyltetrahydrofolate. This medication has been used to prevent and treat this condition for over thirty years 18. It should be kept in mind that each high-dose MTX chemotherapy schedule includes the leucovorin dosing schedule to be administered during treatment cycles. The patient in this case was given 50mg of calcium folinate orally every six hours, but he still developed MTX nephrotoxicity.
Finally, as for glucarpidase, its efficacy in the management of MTX nephrotoxicity has been widely demonstrated 3,9,10,15,16 at doses of 50 U/kg body weight in a 5-minute intravenous infusion, as it reduces the serum concentration of this drug by 97% after 15 minutes of administration. However, it is important to note that it has no effect on intracellular MTX 7,18-20, so management with glucarpidase should include leucovorin, which should never be administered within two hours before or after glucarpidase because it can also cleave leucovorin.
Renal support therapy has also been included in the therapeutic options for MTX nephrotoxicity when other treatment measures are unsuccessful. From a physiological perspective, MTX has a volume of distribution of 0.4-0.8 L/kg, a molecular weight of 454 Daltons and a plasma protein binding of 50%, so extracorporeal renal support therapy has a favorable profile for MTX elimination. However, its use is subject to debate since there is a high level of MTX binding to plasma proteins; therefore, hemoperfusion 21, hemodialysis with high cut-off filters 22, or continuous renal support by means of continuous veno-venous hemofiltration have been proposed 23. These therapeutic options can be considered in addition to the previously mentioned medical management and in cases of persistently high MTX concentrations and multisystem involvement. In the reported case, the patient did not require any extracorporeal renal support therapy because the involvement was limited to the kidney, and the measures implemented resulted in a rapid decrease in MTX levels.
CONCLUSIONS
The use of high-dose MTX is common in chemotherapy regimens. The present case illustrates the usual clinical scenario of nephrotoxicity induced by this drug. It also emphasizes the importance of understanding the mechanisms that cause it and describes the various therapeutic options available for treating it with the aim of improving the overall prognosis of patients.














