INTRODUCTION
In ancient times, the cyclical occurrence of epileptic seizures, just like menstrual cycles, was attributed to lunar phases, but in 1857, Sir Charles Locock, at a meeting of the Royal Medical and Chirurgical Society, first described the relationship between epileptic seizures and the menstrual cycle. Since then, multiple studies on what is known as catamenial epilepsy, a condition that refers to the exacerbation of seizures in association with the menstrual cycle, have been conducted. It is worth mentioning that the term "catamenial" is derived from the Greek word katamenios, which means monthly 1.
Worldwide, it is estimated that 2.4 million people are diagnosed with epilepsy each year and, in high-income countries, new cases per year vary between 30 and 50 per 100 000 inhabitants of the general population, while this figure can be up to 2 times higher in low- and middle-income countries 2. In Colombia, specifically, Velez & Eslava-Cobos 3 reported in 2006 that the overall prevalence rate of epilepsy was 11.3 cases per 1 000 people.
Herzog et al.4, in a study of 184 women with intractable complex partial seizures, found that about one third of the participants may have catamenial epilepsy. In turn, Ducan et al.5, in a study that aimed to establish the incidence of catamenial epilepsy in 40 young women with refractory epilepsy, found that only 5 participants (15.5%) met the criteria established by the authors for defining catamenial epilepsy (occurrence of at least 75% of seizures within 4 days before and 6 days after the onset of menstruation).
Currently, there are multiple therapeutic approaches available, both hormonal and non-hormonal, for the treatment of catamenial epilepsy; however, there is no consensus or specific recommendations regarding its management 6,7. The following is the case of a patient diagnosed with this disorder, who was treated with progestogens and had a satisfactory outcome.
CASE PRESENTATION
A 31-year-old woman of mixed racial descent, from a middle-class household, and born in Bogotá, D.C. (Colombia), was taken to the emergency department of the Hospital Universitario Clínica San Rafael in Bogotá by a relative due to an episode of tonic-clonic seizures directly associated with her menstrual period and the subsequent onset of generalized headache. The patient's relative stated that she experienced the same symptoms every 28 days and that she had a history of refractory structural epilepsy, visual impairment due to meningitis at 9 months of age, autism spectrum, schizophrenia under pharmacological treatment, and surgical sterilization performed for family planning purposes.
On admission to the hospital, management was started with 5mg of intravenous midazolam; however, due to refractoriness to this treatment, the medication was switched to levetiracetam 1g administered intravenously. 36 hours after admission, the patient had a new seizure, which was considered to be refractory status epilepticus. Therapy propofol was initiated at an infusion rate of 100mg every hour, which was maintained for 3 hours until a new episode occurred. Due to this new seizure, rapid sequence intubation was performed and 3mg of midazolam were administered intravenously every hour. She was transferred to the intensive care unit (ICU) where she was assessed by the neurology service to monitor and stabilize her condition, on the one hand, and to determine the advisability of starting treatment with antiepileptic drugs, on the other hand.
On the fifth day of her stay in the ICU, the patient presented with sepsis of urinary origin, for which she received treatment with 4.5g of piperacillin-tazobactam every 8 hours for 7 days; after 4 days she was stabilized and extubated. After 15 days in the ICU, she was transferred to the floor under the care of the internal medicine service, and a consultation with the gynecology and obstetrics department was requested to determine the need for hormone therapy.
Prior to being admitted to the emergency room, the patient was receiving outpatient care from psychiatry, neurology, and gynecology specialists. She was taking 250mg of valproic acid every 12 hours; 200mg, 100mg, and 200mg of lacosamide in the morning, afternoon, and evening, respectively; 5, 5, and 25 drops of clonazepam in the morning, afternoon, and evening, respectively; 250mg of acetazolamide per day; and 5mg of olanzapine per day.
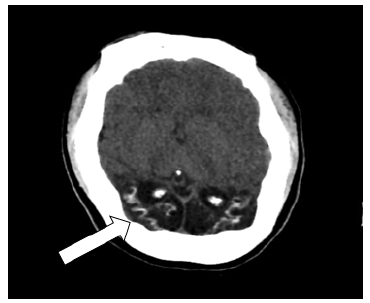
On the same day of admission, the patient underwent a computed tomography scan of the skull (Figure 1) that revealed cortical laminar necrosis in the occipital region, a possible sequela of childhood meningitis, and an electroencephalogram in which no epileptogenic foci were observed.
Source: Document obtained during the study.
Figure 1 Computed tomography scan of the skull with evidence of cortical laminar necrosis in the occipital region.
Since the seizures exacerbated every 28 days and were associated with menstruation, the clinical diagnosis of structural focal epilepsy with catamenial features was established.
The patient's case was presented to a multidisciplinary medical board (internal medicine, neurology and gynecology and obstetrics departments) on February 27, 2020. At this meeting, the advantages and disadvantages of medical pharmacological management versus surgery (bilateral oophorectomy) were discussed and it was decided to start pharmacological treatment with oral progestogens (2mg of dienogest orally daily for 3 months) and to monitor the patient's clinical response, considering that surgery in a young woman would imply early menopause with the associated risks of increased cardiovascular risk and early osteoporosis
Due to the patient's adequate clinical progress and the fact that she did not have any new seizures, she was discharged 21 days after her admission. Three months after starting treatment, she attended a follow-up appointment and was found to have adequate adherence to the treatment and complete remission of epileptic seizures had been achieved. Her relative reported no adverse effects.
DISCUSSION
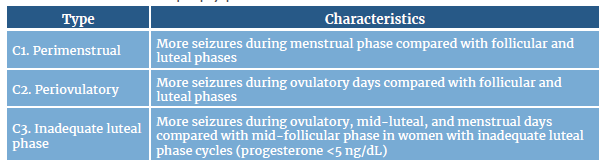
Catamenial epilepsy refers to the worsening or exacerbation of epileptic seizures related to hormonal changes during the menstrual cycle 1. This type of epilepsy develops because certain times during the menstrual cycle increase the likelihood of having epileptic seizures, a phenomenon known as seizure patterns. The patterns of catamenial epilepsy, which were described by Herzog et al.4 in 1997, are related to hormonal variations, as shown in Table 1.
Catamenial epilepsy is not a distinct type of epilepsy, but rather a catamenial exacerbation of epileptic seizures that occurs frequently in multiple types of epilepsy 8 and is difficult to diagnose. In the case presented here, the main obstacle to determining the cause of the epileptic seizures was the difficulty in communicating with the patient and her multiple comorbidities; however, the refractoriness to treatment with antiepileptic medication 9 and the cyclical onset of epileptic seizures associated with her menstrual period were useful to establish the diagnosis because increased seizure frequency associated with some phase of the menstrual cycle is considered a diagnostic criterion of catamenial epilepsy 10.
Catamenial epileptic seizures can occur in patients with any type of epilepsy but are more frequently seen in those with temporal lobe epilepsy 2. It is important to bear in mind that catamenial epilepsy is a common form of epilepsy refractory to drug therapy in women and it may have a significant impact on the quality of life of these patients 6,11.
The pathophysiology of the influence of sex hormones on epileptogenesis is poorly understood; however, catamenial epilepsy is thought to occur due to the neuroactive properties of endogenous steroid hormones and the natural cyclic variation in their serum levels throughout the menstrual cycle 7.
The onset of epileptic seizures has been correlated with changes in progesterone, estrogen, and neurosteroid levels: estrogen levels increase in the follicular phase, with a peak at the time of ovulation, while progesterone levels increase after ovulation and decrease before the end of the menstrual cycle 6,11. These two hormones modulate neuronal excitability through their derivatives, which are known as neurosteroids, via gamma-aminobutyric acid (GABA) receptors 11.
There is strong evidence in animal models that the physiological actions of progesterone are mediated by receptors belonging to the nuclear receptor superfamily that act as transcription factors. Thus, Reddy et al.12 and Reddy & Rogawski 13 found that the anticonvulsant effects of progesterone are associated with the reduced 5a metabolites in this hormone; however, these decreased levels also affect susceptibility to epileptic seizures.
Progesterone reduces neuronal excitability by intervening in different processes: it enhances the action of adenosine, which exerts a powerful inhibitory function on neuronal activity; it decreases cytoplasmic estrogen receptors, altering the plasticity and excitatory capacity of neurones; and reduces the conductance of the nicotinic acetylcholine receptor, altering plasma membrane depolarization 10. Nevertheless, the antiepileptic effect of this hormone is mainly attributed to its conversion to 3a-5a-tetrahydroprogesterone (3a-5a-THP), which increases the chloride current to the intracellular space induced by GABA. Furthermore, in addition to influencing the responses of this acid, progesterone and its metabolites also impact its excitatory mechanisms and, therefore, alter the composition of GABA-A receptor subunits by dynamically changing the GABA receptor subunit composition in situations of progesterone withdrawal, which occurs during the premenstrual stage. Seizure threshold and sensitivity to anticonvulsant drug therapy also vary cyclically 14.
On the other hand, the role of estrogens is complex but possibly neuroexcitatory in certain circumstances 15. Estradiol, for example, may affect neuronal excitability by cytosolic neuronal estrogen receptor-mediated, genomically dependent mechanisms; moreover, because they regulate the expression of genes that affect the activity, release, and postsynaptic action of different neurotrans-mitters and neuromodulators, estrogens may increase the excitability of neurons that concentrate estradiol 16. Evidence has also been found that estradiol increases the sensitivity of the N-methyl-D-aspartate (NMDA) receptor to glutamate and that this increased sensitivity positively correlates with increased dendritic spine density in hippocampal CA1 pyramidal cells 17.
It has been established that 5a-reduced neurosteroids (progesterone metabolites) are responsible for protection against epileptic seizures caused by progesterone. Therefore, susceptibility to epileptic seizures is very low during physiological conditions associated with high progesterone levels 11.
Progesterone is metabolized by glial cells in the brain, where it is converted to neurosteroids, which can alter both inhibitory and excitatory neurotransmitters 11. It is worth noting that the most studied neurosteroids are allopregnanolone, allotetrahydrocorticosterone and androstanediol 10. Neurosteroid levels in the brain depend on the amount of circulating steroid hormones since the enzymes involved in their synthesis are expressed in much of the brain, mainly in the cortex, hippocampus, thalamus, amygdala, and hypothalamus; however, GABAergic interneurons do not possess these enzymes. It is also believed that endogenous neurosteroids can regulate GABA activity and release 11 and that the activation of the GABA-A receptor by several ligands leads to an influx of chloride ions and to a hyperpolarization of the membrane that dampens neuronal excitability 9.
Catamenial epilepsy is diagnosed based on a detailed medical history, hence the importance of recording the anticonvulsant drugs used by patients and, in addition, asking them to keep a "seizure diary" under specific instructions from the treating physician 18 because it allows to identify the patterns of epileptic seizure occurrence. Nevertheless, there are different ways to determine whether patients are ovulating or not, for example by measuring basal body temperature in the morning (upon awakening), where an increase of at least 0.021°C is an indicator of ovulation.
Another way to determine whether patients are ovulating is through a kit to measure luteinizing hormone (LH) in urine, which helps detect the surge in luteinizing hormone 32 to 36 hours before ovulation. These tests should be performed depending on the length of the menstrual cycle: if the cycle is 28 days long, they should be done on day 12 and for 10 consecutive days until the LH peak occurs.
More sophisticated measures to confirm ovulation include documentation of a mean serum progesterone concentration >3ng/mL and a decrease in dominant follicle volume ≥90% as measured by transvaginal ultrasound or secretory endometrial biopsy 1.
According to Kandeepan & Shaaban 19, the treatment of catamenial epilepsy is mainly directed toward hormonal therapy, as it has been found that this type of epilepsy does not usually improve with antiepileptic drugs alone, as evidenced in the present case. Similarly, it has been hypothesized that progesterone, progesterone metabolites, or estrogen antagonists can be used in combination with current antiepileptic drugs to treat patients with inadequate luteal phase or with anovulatory cycles 7.
Specifically, two approaches to progesterone therapy have been proposed: cyclic progesterone therapy, which involves supplementing progesterone during the luteal phase and gradually withdrawing it during the premenstrual phase; and suppressive therapy, which involves suppressing the menstrual cycle through the use of injectable progestins or gonadotropin-releasing hormone analogues 16.
Najafi et al.20 conducted a double-blind randomized controlled trial in 38 women with intractable catamenial epilepsy, in which patients were divided into two groups, a case group that, in addition to antiepileptic drugs, received two 40mg of progesterone tablets (megestrol) in the second half of the 15 to 25-day cycle, and a control group that, in addition to the antiepileptics, received two placebo tablets daily. In this study, the authors found that the degree of reduction in the number of seizures in the case group was greater than in the control group, being statistically significant (p<0.05).
Regarding progestin therapy, Mattso et al.21 reported in their study that the use of parenteral medroxyprogesterone acetate may reduce the frequency of seizures when administered in sufficient doses to induce amenorrhea. However, as indicated by Herzog 16, it is not clear whether the effect is related to the direct anticonvulsant activity of medroxyprogesterone or to the hormonal consequences of induced amenorrhea. Likewise, treatment with synthetic gonadotropin-releasing hormone (GnRH) analogs has been shown to have anticonvulsant effects in patients with intractable catamenial epilepsy 6.
In the management of catamenial epilepsy, triptorelin therapy has been shown to be more effective for the treatment of patients whose seizures are limited to the perimenstrual period 22. Furthermore, the use of clomiphene, an ovulation stimulant used to treat infertility in women with oligoanovulation or anovulation, 7 has shown a reduction in the frequency of epileptic seizures in this type of patient 23. In turn, ganaxolone, a neurosteroid analog whose mechanism of action modulates GABA-A receptor activity and has proven anti-convulsant properties, has been shown to be the most reliable therapy and the one that least exposes patients to the risk of hormonal side effects 24.
Another therapeutic approach is non-hormonal therapy, which was initially based on acetazolamide. Its mechanism of action is not well known; however, it is clear that its use develops tolerance, resulting in a decrease in efficacy over time. Therefore, this drug can only be administered intermittently, which, although appropriate for catamenial epilepsy, is not ideal for prophylaxis of ordinary epileptic seizures 7.
The overall effectiveness of benzodiazepines for seizure clusters has led to accept its intermittent use as an approach to catamenial seizure management; however, the only benzodiazepine studied for this purpose to date is clobazam 25.
Surgical management in the treatment of catamenial epilepsy is not common, but it has been used as a menstrual cycle suppression strategy that favors the decrease of sex hormones and, consequently, decreases epileptic seizures with catamenial features. In this regard, Vilos et al.26 reported the case of a woman with abnormal uterine bleeding who developed catamenial neurological signs and symptoms (including seizures) and in whom computed tomography scans and magnetic resonance imaging demonstrated a circumscribed lesion in the left centrum semiovale of the brain. The patient was treated with a GnRH agonist (goserelin) for 3 months and a subsequent laparoscopic bilateral oophorectomy, which resolved the neurological symptoms completely.
CONCLUSIONS
Catamenial epilepsy is a disorder of clinical importance that should be considered as a cause of epilepsy refractory to antiepileptic therapy. Its approach should be multidisciplinary, and its treatment should focus on improving the quality of life of patients. Therefore, the importance of obtaining an adequate medical history that compiles relevant information for the management of patients suffering from this disease cannot be overstated.
With regard to treatment, it is important to bear in mind that each patient must be individualized since studies have supported certain combinations of hormonal and non-hormonal treatments, along with anticonvulsants, so it is essential to identify the specific pattern of catamenial epilepsy; however, surgery is an option that can be considered if there is no improvement with pharmacological treatment.