INTRODUCTION
Pseudohypoparathyroidism (PHP) is a rare endocrine disease characterized by resistance to the action the parathyroid hormone (PTH). Its prevalence worldwide is unknown, but in Denmark and Japan, according to studies reviewed by Mantovani et al.,1 it is 1.1 and 0.34 cases per 100 000 inhabitants, respectively. In Colombia, according to the Ministry of Health 2, this is a rare or orphan disease and is therefore expected to have a prevalence of <1 case per 5 000 people.
The first report of PHP was made in 1942 by Fuller Albright, who described 3 cases of patients with osteodystrophy, hypocalcemia and hyperphosphatemia that did not improve with the administration of PTH; these patients also had altered excretion of nephrogenous cyclic adenosine monophosphate (cAMP) and urinary phosphate levels 3. This condition, as pointed out by Carli et al.4, was called "pseudohypoparathyroidism" because PTH levels were elevated in the presence of inappropriately low serum calcium and elevated serum phosphorus levels.
The PHP nomenclature has evolved over time to keep pace with molecular technological advances in order to improve its clinical characterization. Currently, there are 3 main subtypes of PHP: 1) PHPlA, caused by a loss-of-function maternal-effect mutation in the GNAS coding sequence; 2) PHP1B, caused by a methylation defect in the GNAS coding sequence; and 3) pseudo-pseudohypo-parathyroidism (PPHP), caused by a loss-of-function paternal-effect mutation in the GNAS coding sequence. There are also some other related disorders, including acrodysostosis and progressive osseous heteroplasia.
Although the different subtypes of PHP have common molecular mechanisms, the clinical presentation and severity of the disease can vary considerably among affected individuals, even among patients carrying the same genetic alteration 1. In patients with PHP, some features suggestive of the disease such as early-onset obesity, neurodevelopmental delay, or transient hypothyroidism may be observed during the first months of life. However, in the absence of a family history or other typical signs, such as ectopic ossifications, diagnosis may take several years due to a failure to recognize the disease.
During childhood, other manifestations such as stunted growth, brachydactyly or hypocalcemia (causing neuromuscular symptoms or even seizures) often lead to further examination to determine the etiology, which generally results in a late diagnosis of this disease 5,6. In this sense, once a case of PHP is suspected, a molecular analysis should be performed to achieve a specific diagnosis, which will allow establishing an appropriate approach based on the prevention of complications, lifestyle adjustments, and treatment of endocrine deficits 5,7.
Although there is no specific treatment for PHP, it should be directed to correct alterations in phosphate and calcium metabolism to avoid complications arising from this disorder, in addition to maintaining an adequate blood calcium level through the formulation of calcium carbonate or calcium citrate. To ensure adequate absorption of this element, and due to the resistance to the action of PTH, activated forms of vitamin D, such as calcitriol, should also be administered, adjusting the dose to achieve normal calcium levels and avoid hyperphosphatemia secondary to the use of this vitamin, which will decrease serum PTH levels and prevent bone demineralization.
On the other hand, if patients with PHP present with thyrotrope axis or gonadotrope axis resistance along with growth hormone (GH) resistance, they should be treated with levothyroxine and sex hormones, respectively. Likewise, in order to control obesity in PHP patients, it is essential that they adopt proper eating habits and exercise regularly 8.
CASE PRESENTATION
An 18-year-old male, mestizo, born and living in Bogotá (Colombia), went with his mother to an outpatient endocrinology clinic at a tertiary care hospital in Bogotá. He was referred due to a seizure episode and phosphate and calcium metabolism disorders detected during a hospital stay two months earlier. The patient was a business administration student, son of healthy non-consanguineous parents, and delivered preterm at 32 weeks, with birth weight and height of 2 700g and 49cm, respectively.
His medical history included cryptorchidism from birth, which was corrected surgically at the age of 10 years. When interviewed, neither the patient nor his mother reported any significant family history, but the mother stated that from the first days of life, her son had difficulties with sucking and effective breastfeeding and that he subsequently had a rapid weight gain with the appearance of childhood obesity, short arms, and short stature (Figure 1).
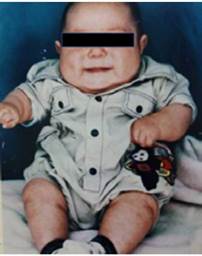
Source: Image obtained while conducting the study.
Figure 1 Patient at the age of 3 months with evidence of rounded facies, childhood obesity, and short limbs.
At 4 months of age, the patient was diagnosed with hypothyroidism and started treatment with levothyroxine; however, as time went by, dysmorphic features, rounded facies and brachydactyly became evident. During his preschool years, he had multiple upper respiratory tract infections that required hospitalization for treatment. At the age of 9, he presented a pathologic fracture of the left radius.
During his adolescence, the patient began to experience symptoms in several systems: paresthesia in extremities, chronic constipation, recurrent epistaxis, and low back pain. No neurocognitive alterations were observed during his growth. At the age of 14 years, hypocalcemia, hyperphosphatemia and elevated PTH levels were documented for the first time; 5 years later, he had a seizure for which he consulted the emergency department.
On admission to the emergency department, a Holter monitoring study was requested, which identified sinus bradycardia of up to 30 beats/minute. Blood biochemistry tests were also performed, showing elevated levels of PTH (409.7 pg/mL, reference value of 10-55 pg/mL) and phosphorus (5.99 mg/dL, reference value of 2.84.5 mg/dL), as well as low levels of calcium (7.81 mg/dL, reference value of 8.5-10.2 mg/dL) and 25-hydroxy vitamin D (17.6 ng/mL, taking into account a cut-off point of deficiency values <20 ng/mL). Finally, a CT scan of the skull showed calcifications in the periventricular white matter adjacent to the right temporal horn and in the perimesencephalic cistern, as well as normal cortical thickness for age in the cranial vault (Figure 2).
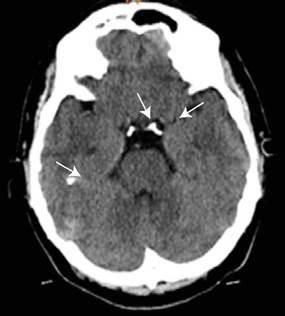
Source: Image obtained while conducting the study.
Figure 2 CT scan of the skull showing calcifications in the periventricular white matter adjacent to the right temporal horn and in the perimesencephalic cistern.
The patient had a satisfactory response, with resolution of bradycardia after intravenous administration of calcium gluconate and oral administration of calcium carbonate 1 200mg per day, and no new seizure episodes. Consequently, he was discharged 7 days after being admitted to the emergency room with an indication for an outpatient endocrinology consultation.
During the assessment by the endocrinology service and taking into account the data collected in his medical record, a detailed physical examination was performed, in which the patient's short stature and brachydactyly stood out. The patient weighed 58kg, was 150cm tall, and had a body mass index of 26.1 kg/m2 (Figure 3).
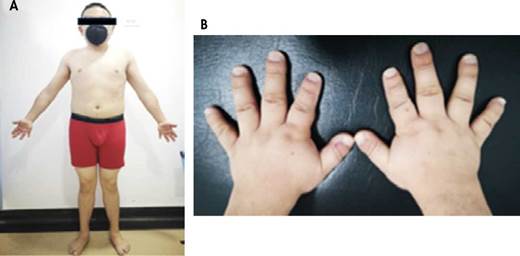
Source: Images obtained while conducting the study.
Figure 3 Patient at 18 years of age during his visit to the endocrinology service. A) anterior view photograph showing short stature and short extremities; B) photograph of the hands showing brachydactyly.
In view of the findings, complementary blood biochemistry and imaging studies were requested. In a follow-up appointment with the endocrinology service (approximately 2 months after the first assessment), it was found that bone densitometry showed a z-score corrected for age and sex of -2.1, both in the lumbar region of the spine and in the neck of the femur, and that the spine X-ray showed biconcave fragility fractures at L3 and L4, which could explain the low back pain that the patient was experiencing. Likewise, X-rays of long bones showed osteochondromas in the fibula and right forearm (Figure 4).
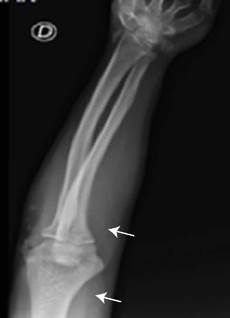
Source: Image obtained while conducting the study.
Figure 4 X-ray of the right humerus showing radiopaque lesion with bone demineralization involving the lateral cortex and the metaphysis and epiphysis of the distal humerus and lateral epicondyle, which could indicate an osteochondroma.
Complementary tests showed that the patient had elevated thyroid stimulating hormone (TSH) levels (7.54 IU/mL, reference value 0.4-4.5 IU/mL) but normal free thyroxine (T4L) levels (0.96 ng/dL, reference value 0.9-2.3 ng/dL) taking into account that at that time he was receiving levothyroxine supplementation of 100mcg per day. Moreover, adequate levels of gonadotropins -follicle stimulating hormone (FSH) at 4.23 IU/mL (reference value 1.5-12.4 IU/mL) and luteinizing hormone (LH) at 3. 22 IU/mL (reference value 1.7-8.6 IU/mL) -, total testosterone (5.41 ng/mL, reference value 2.6 to 15.9 ng/mL), and somatomedin C (205.2 ng/mL, reference value 133-430 ng/mL) were observed. Testicular ultrasound showed microcalcifications in both testicles, one of 7.6mm in the left testicle and another of 6.2mm in the right testicle.
Based on the findings, the patient was referred for genetic testing under the diagnostic suspicion of PHPlA. During that assessment, performed one month later, it was decided to request the sequencing of the GNAS gene, which revealed a heterozygous pathogenic variant (cl65_l66insTCAT) implying that there is a pathogenic insertion of the nucleotides thymine, cytosine, adenine and thymine (TCAT) between position 165 and 166 of the coding DNA of the GNAS gene. Furthermore, at the protein level, this variant causes a change of isoleucine to serine at position 56, leading to a reading frame shift with a premature stop codon at position 67 and, finally, a protein of only 1 037 amino acids (peptide form p.Ile56Serfs*12).
One month later, the patient was evaluated again by the endocrinology service with the results of the genetic study, which showed an abnormality of the alpha subunit of the G protein coupled to the PTH receptor with alteration of the downstream signaling pathway, so treatment was adjusted with oral calcium carbonate 6oomg every 4 hours, oral calcitriol 0.5mcg every 8 hours, and oral aluminum hydroxide 5mL as a phosphorus chelator with each meal, all indefinitely. Likewise, the dose of levothyroxine was titrated by increasing the total weekly dose by 25%, i.e., 125mcg orally per day. Joint assessments by the nutrition and nephrology departments were requested, and first-degree relatives were referred to genetic counseling, but no similar cases were found within their family tree.
Since then, the patient has been followed up by the endocrinology specialist and the aforementioned specialties at intervals of 3-5 months, with an adequate therapeutic response. At his last follow-up, PTH levels had improved (185 pg/mL), phosphorus levels had decreased (5.09 mg/dL), calcium levels were within normal limits (9.6 mg/dL), and 25-hydroxy vitamin D levels had increased (23.1 ng/mL). These tests were performed approximately one year after the first endocrinology consultation.
DISCUSSION
PHP is a rare cause of congenital phosphate and calcium metabolism disorders, and its diagnosis is usually difficult and delayed because the clinical manifestations may be insidious and appear at an advanced age 1. PHP was first described in 1942 by Albright and colleagues, who at that time defined it as a disease characterized by somatic and developmental anomalies, including subcutaneous heterotopic ossifications and brachydactyly. A decade later (1952), Albright identified a patient with the same clinical phenotype, but with normal calcium, phosphate and PTH levels; he called this condition "pseudo-pseudohypoparatidoidism" 9, while the set of skeletal defects described (rounded facies, short stature, obesity, subcutaneous ossification, short fourth metacarpal, etc.) was named Albright hereditary osteodystrophy (AHO). The latter was subsequently associated with altered chondrocyte and osteoblast differentiation and early closure of growth plates 1,10.
PTH resistance in the absence of AHO phenotypes is the main feature of PHPlB patients. However, recent studies have characterized other related phenotypic findings, such as intrauterine growth restriction in PPHP cases and development of early-onset obesity, frequent respiratory and ENT infections, delay in reaching developmental milestones, and cognitive impairment in PHPlA patients 5,11.
PHP and other related disorders arise from a defect in the cAMP and PTH and PTHrP receptor signaling pathway. Both PHPlA and PPHP are caused by heterozygous variants affecting exons 1-13 of the GNAS gene, which encodes the alpha subunit of the stimulatory G protein (Gas) that couples receptors for many hormones and neurotransmitters by activating the adenylate cyclase enzyme 11,12. Thus, the disease phenotype is associated with alterations in maternal inheritance in the case of PHP1A, or paternal inheritance in the case of PPHP, which is explained by genomic imprinting phenomena. Thus, patients with PHP1A have resistance to multiple Gas-coupled hormones such as PTH, FSH, LH, TSH and gonadotropin-releasing hormone, with presence of early-onset obesity. On the other hand, patients with PPHP have AHO phenotypes without obesity or any other characteristic hormone deficiencies 4,5,13. PHP1B is characterized by defects in methylation of the GNAS locus. The main features of each PHP subtype are described in Figure 5.
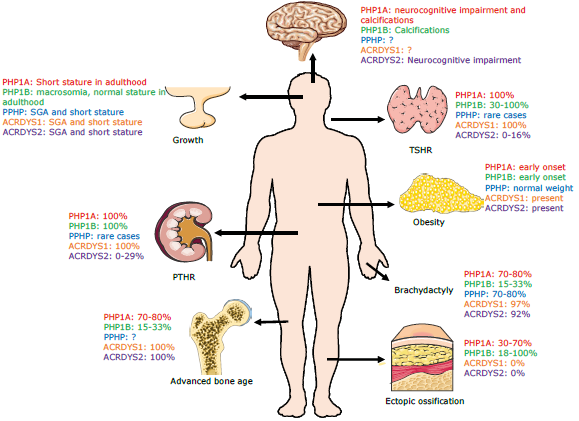
ACRDYS: acrodysostosis; SGA: small for gestational age; ?: not well-defined clinical manifestations; TSHR: thyroid stimulating hormone receptor; PTHR: parathyroid hormone receptor.
Source: Elaboration based on Carli et al. (5).
Figure 5 Clinical features of PHP and related disorders.
The term PHP1C was initially used to classify the patients who had the PHP1A phenotype and normal biochemical Gas activity due to mutations of the molecular Gas-coupled receptor domains and alterations in the catalytic activity of the adenylate cyclase enzyme 14. This designation is no longer used, and these patients are classified as PHP1A. Recently, new molecular techniques have identified other disorders similar to PHP, such as acrodysostosis, which are caused by defects in genes involved in the coupling of Gas and different signaling pathways (GNAS, PRKAR1A, PDE4D, among others) 15.
As with any hypoparathyroidism, hyperphosphatemia leads to increased levels of the calcium-phosphorus product, which induces ectopic calcifications as revealed in the CT scan of the basal ganglia of the patient reported here 16. In cases of PHP, the various hormonal resistances (such as to thyroid hormones, sex hormones, etc.) are driven by the expression of the maternal gene in tissues such as the proximal renal tubule, thyroid, pituitary, gonads, etc.; however, gene function is not always altered homogeneously in all tissues, causing hormonal resistances with varying degrees of frequency and in a polymorphous manner throughout the patient's life 17. TSH resistance is by far the most frequent, which is consistent with the present case, where the patient was diagnosed at 4 months of age with hypothyroidism that progressed to possible subclinical hypothyroidism as part of the natural history of resistance to this hormone that occurs in cases of PHP.
Thus, in the present case, a diagnosis of hypothyroidism secondary to TSH resistance is suggested, excluding the diagnosis of congenital hypothyroidism; however, the presence of autoimmune thyroiditis could also be considered as a possible cause of hypothyroidism although with a lower probability. In this patient, nevertheless, thyroglobulin and anti-thyroglobulin antibody levels were not evaluated, which is a limitation of this case report 18.
Contrary to isolated TSH resistance due to mutations of its receptor, where other hormone resistances are not found and the typical dimorphisms of PHP are not observed 19, in PHP1A, TSH levels are slightly increased and remain stable over time. Furthermore, iodine uptake is not compromised and ultrasound scans show that the size of the thyroid gland is normal or slightly decreased.
A noteworthy aspect of the case presented is that, although the literature reports neurocognitive alterations in cases of PHP1A, this patient had an apparent adequate neurological development since he was pursuing a university degree. However, it should be noted that no psycho-technical tests were conducted to measure his IQ. Similarly, this case highlights the fact that cryptorchidism, which was treated surgically in infancy with subsequent ultrasound confirmation of age-appropriate testicular size, was probably caused by partial gonadotrophin resistance as part of the clinical manifestations of PHP1A.
The patient's short stature can be explained by several factors: 1) that it is part of the PHP1A syndromic complex, 2) that it is due to a partial resistance to GH action, 3) that it is a clinical manifestation of hypothyroidism diagnosed at such a young age, and 4) that it is due to the presence of mild hypogonadism secondary to a resistance to gonadotrophin or caused by cryptorchidism, which may have affected testicular function.
On the other hand, the causes of severe osteoporosis in the patient include vitamin D deficiency, the presence of mild hypogonadism for the reasons previously discussed, and resistance to the action of GH. In turn, the seizure episode could have been triggered by several factors such as symptomatic bradycardia phenomena, hypocalcemia, and basal ganglia calcifications.
When searching for the genetic variant found in the patient (c165_166insTCAT of the GNAS gene) in the different databases, no report of this mutation was found. Thus, the case presented here refers to PHP1A (the most frequent type of PHP) caused by a pathogenic variant described for the first time in one of the alleles of the GNAS gene and of autosomal dominant inheritance, with a heritability risk of 50% for offspring. Although this classification is based on some key points of the pathophysiology of the disease, the response to exogenous PTH and the in vitro activity of Gas (taking into account the presence of AHO), it excludes other types of mutations with recently discovered molecular mechanisms that cannot be assigned to the previous classification or classifies them inaccurately.
New classifications, such as that of the EuroPHP network, which collectively labels PHP types as inactivating disorders of PTH/PTHrP signaling, may help the clinician better understand the disease and its subtypes 11,20. However, there are currently no clinical practice guidelines for the management of PHP, although it should be kept in mind that there is an expert consensus summarizing the clinical and paraclinical findings and care recommendations for this type of patient, which the reader is advised to review 5.
In Latin America, few cases of PHP have been reported in countries such as Brazil 21 and Chile 22. In Colombia, the first report was made in 1957 and describes the case of three siblings with AHO phenotype, hypocalcemia, and no response to PTH 23. More recently, Trejo et al.24 retrospectively reviewed the endocrinology databases of two tertiary care centers in Medellín from 2012 to 2016, and found 4 cases of patients with PHP, 2 of them with PHP1B/2 and the other 2 with PHP1A/1C, all diagnosed in adulthood.
The present case illustrates how the same condition has a wide spectrum of clinical manifestations that can change with age and vary over a lifetime. In this sense, to make an effective diagnosis of PHP, it is necessary to perform a detailed review of the patient's medical records and to focus on their personal history. In addition, physical examination is critical, as the characteristic dysmorphic findings mentioned above may lower the threshold for diagnostic suspicion in cases with a characteristic phenotype.
CONCLUSIONS
A case of PHP1 is reported here along with its clinical laboratory and imaging features and genetic study findings, from which it can be established that PHP comprises a spectrum of complex and under-recognized endocrine metabolic disorders with heterogeneous and progressive clinical manifestations that can lead to potentially fatal consequences in the absence of timely diagnosis and treatment. Therefore, ongoing multidisciplinary medical follow-up is required in patients suspected of having this disease.














