Introduction
Diabetic nephropathy (DN) is one of the main complications of diabetes mellitus (DM). It is estimated that between 20 and 40% of patients with DM develop it, this being the main cause of chronic kidney disease (CKD) in advanced stage, worldwide1. Historically, albuminuria has been fundamental for the diagnosis and clinical staging of the DN. In recent years, the presence of three new groups of kidney diseases present in diabetics has been observed. The first constituted by diabetics with progressive loss of glomerular filtration rate (GFR) without the presence of albuminuria, called patients with non-albuminuric diabetic kidney disease (DKD-NA). The prevalence of this disease2-4 varies between 25% and 50% of patients who have a GFR less than 60 ml/min 1.73mt2. Patients with DKD-NA present different characteristics to the albuminuric population, such as: higher proportion of female sex, older age, lower GFR, good control of blood pressure, non-Hispanic white race, lower prevalence of smoking and higher prevalence of polyneuropathy5-7.
The second group includes diabetics with non-diabetic kidney disease (NDKD). This includes a great diversity of pathologies that affect the GFR and that do not meet the histological criteria of ND, with or without proteinuria. It has been reported that its prevalence in studies varies from 14% to 82, 9% 8.
The last group is represented by diabetic patients with diabetic base nephropathy and non-diabetic superimposed condition. This causes worsening of renal function8.
However, it is important to note that there are no studies where an adequate diagnostic flow chart has been performed, including a renal biopsy, and that allows the diagnosis of kidney, diabetic or non-diabetic, non-macroalbuminuric disease to be identified with certainty.
In order to establish, in our environment, which of the three groups is present, this study was advanced in patients with long-standing type 2 DM, not macroalbuminuric, with deterioration in renal function. Their clinical and etiological characteristics were identified, with possible impact on their prognosis and long-term behavior.
Subjects and methods
We reviewed a total of 102 medical records of patients older than 18 years, type 2 diabetics, more than 10 years after being diagnosed, located in the city of Manizales, Caldas, Colombia. They presented elevation of nitrogen compounds of unclear origin, no macro-albuminuria (proteinuria less than 300 mgs/24 hours), attended by the researchers in the outpatient program of the RTS Caldas Renal Unit during the period from 2008 to 2016, without admission to the dialysis program. The exclusion criteria were: patients with previously documented proteinuria greater than 300 mg in 24-hour urine; hospitalization in the last 2 months; recent presence of diabetic decompensation (diabetic ketoacidosis or hyperglycemic hyperosmolar state); Secondary DM; documented malignancy at the time of renal biopsy; advanced kidney disease at the time of the study, or with rapid decline in renal function over the course of 3 months (rapidly progressive); and, finally, immunological profile compatible with primary or secondary nephropathy.
According to the above, 35 patients were excluded: 17 for incomplete studies, 11 lost during follow-up, 3 were admitted to hemodialysis-type renal replacement therapy, 1 to peritoneal dialysis, 3 patients had proteinuria greater than 300 mg in 24 hours, detected in previous years when reviewing medical records. In total, 67 patients finally met the inclusion criteria for this case series study.
The 67 patients who met the inclusion criteria underwent bilateral renal and urinary tract ultrasound and renal artery Doppler ultrasound. If the previous studies were inconclusive for the diagnosis, we proceeded to perform a percutaneous renal biopsy under ultrasound guidance. Only angioresonance of renal arteries was requested when there was a high index of suspicion of any alteration identifiable by this procedure. The criteria applied for the diagnosis of hypertensive nephropathy were: hypertension of more than 10 years of evolution, with or without microalbuminuria, and renal ultrasound, in which slightly diminished size kidneys were detected, with an increase in cortical echogenicity and decrease in differentiation of the renal sinus and parenchyma9. In the renal vessel Doppler, the diagnosis of chronic ischemic nephropathy (intra-renal microvasculature disease) was considered present when the resistance indices were higher than 0.80 in the inter-lobar arteries and the angioresonance ruled out renal artery stenosis10. This was diagnosed by angioresonance or arteriography of renal arteries; obstructive nephropathy in the presence of unilateral or bilateral hydronephrosis, with or without prostatic hypertrophy.
For the histological classification of diabetic nephropathy, we used the recommendations of the Research Committee of the Renal Pathology Society11 and, for hypertensive nephropathy, findings similar to those described by Helmchen and, more recently, by Wang et al. 12,13. When the findings showed significant mesangial and vascular involvement, diabetic and hypertensive mixed nephropathy was diagnosed14.
The project was approved by the Ethics Committee of the University of Caldas and research and ethics committees of RTS Colombia.
The information was collected using a database designed in Excel, which was completed by the researchers and processed by the statistical program SPSS 15.0 and in Spanish, with a license to the University of Caldas. For the statistical analysis, measures of central tendency and dispersion (mean, standard deviation) were used in the quantitative variables. In the qualitative, proportions.
Results
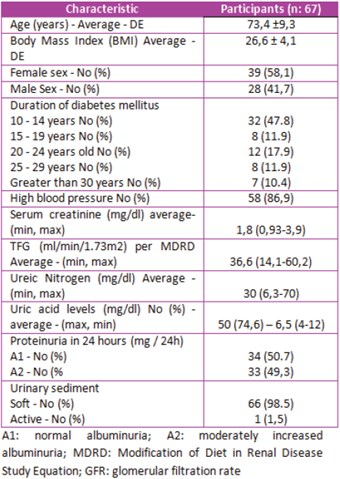
Within the general characteristics of the population Table 1), we found an average age of 73.4 years with an SD ± 9.3; body mass index (BMI) with an average of 26.6 kg/m2, which corresponds to overweight with an SD ± 4.1. According to the duration of DM type 2, 47.8% of patients were between 10 to 14 years old, 17.9% between 20 to 24 years old, 11.9% between 15 to 19 years old and 25 to 29 years old. Finally, 10.4% with more than 30 years of evolution of the disease. With respect to HT, it was found that 86.9% of the participants had this comorbidity.
For laboratory variables, creatinine was detected with an average of 1.8 mg/dL as a minimum value of 0.93 and a maximum value of 3.9. The glomerular filtration rate per mean MDRD was 36.6 ml/ min/1.73 m2, with a minimum value of 14.10 ml/ min/1.73 m2 and a maximum of 60.20 ml/min/1.73 m2. BUN levels, with an average of 30 mg/dl, with a minimum value of 6.3 and a maximum value of 70. With respect to uric acid levels, it was recorded in 74.6% of participants with an average of 6.5 mg/ dl, with a minimum value of 4 and a maximum value of 12.
The urinary sediment was soft in 98.5% of the patients and, in the measurement of proteins in urine of 24 hours; it was found that 50.75% had normal albuminuria (A1). In addition, in 49.3% moderately increased albuminuria (A2), according to the classification recommended by the KDIGO guidelines15.
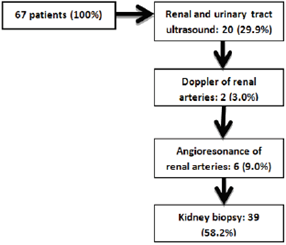
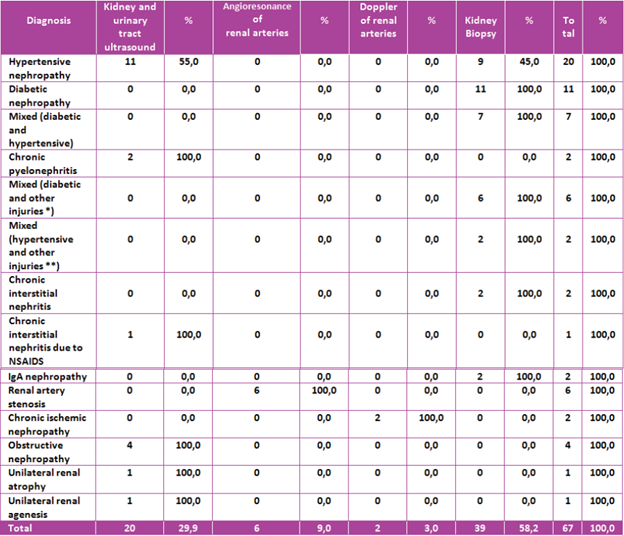
Of the total of 67 participants in the study (figure 1), 28 were diagnosed by imaging studies. By renal and urinary tract ultrasound, 11 patients with hypertensive nephropathy, 4 obstructive nephropathies, 2 chronic pyelonephritis, 1 chronic NSAID nephritis, 1 unilateral renal atrophy, and 1 unilateral renal agenesis were identified. The renal vessel Doppler identified 2 patients with chronic ischemic nephropathy. Finally, by angioresonance of renal arteries, 6 patients with renal artery stenosis, unilateral in 5 patients and, in 1 patient, bilateral. On the other hand, 39 patients (58.20% of the population) underwent renal biopsy, given that by the previous methods a satisfactory diagnosis was not achieved.
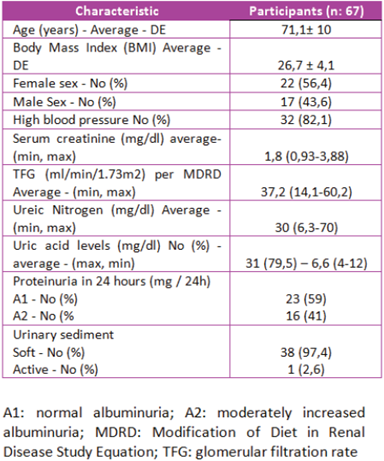
The general clinical characteristics of patients undergoing renal biopsy are described in Table 2. In general terms, it is very similar to that of the total population of participants in the study.
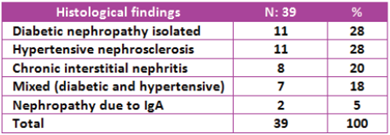
The histological findings were: isolated diabetic nephropathy in 28% of the cases (11 patients); hypertensive nephrosclerosis, 28% (11 patients); chronic interstitial nephritis, 20% (8 patients); mixed, diabetic and hypertensive nephropathy, 18% (7 patients); and IgA nephropathy in 5% of cases (2 patients) (Table 3).
The consolidated diagnosis obtained by imaging and renal biopsy can be reviewed in (Table 4).
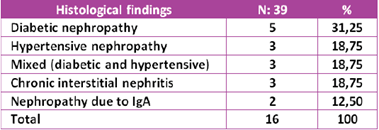
If we analyze, in a separate group, only patients with A2 albuminuria category, together with the findings in renal biopsy, we find that 31.2% (5 patients) correspond to pure diabetic nephropathy, 18.75% (3 patients), to hypertensive nephropathy, 18.75% (3 patients) to mixed nephropathy (diabetic and hypertensive), 18.75% (3 patients) to chronic interstitial nephritis, and 12.5% (2 patients) to IgA nephropathy (table 5).
Discussion
Diabetic nephropathy (DN) classically has been considered an entity that attends a series of stages that include the appearance of albuminuria in various concentrations, with subsequent alteration in GFR. This has been the basis for its classification into 5 stages16. Although this classification emerged for type 1 diabetes, it is assumed that diabetes type 2 with renal involvement occurs in similar stages. It is likely that patients with diabetes mellitus type 1 have renal impairment, mainly due to diabetic nephropathy. In favor of this is the study by Caramoni et al., which reports the results of 23 renal biopsies performed on type 1 diabetic patient with low GFR, who presented DKD-NA. In all cases, advanced diabetic glomerular changes were found17. In contrast, the etiology of DKD-NA in type 2 diabetics could be more variable. Unfortunately, although the DKD-NA in type 2 diabetics has been described in a great variety of studies, they differentiate between clinical characteristics and habits between their normoalbuminuric conditions vs. microalbuminuria or macroalbuminuria without resorting to renal biopsy to obtain a definitive diagnosis2-7.
The NDRD in diabetics, associated or not with a diabetic nephropathy of base, is one of the conditions, found in different studies, in which the renal biopsy has had a definitive role in the final diagnosis.
Ghani et al. performed biopsies on 31 patients with DM, in whom the classic criteria of DN were not met (patients with microscopic hematuria, proteinuria without retinopathy, accelerated renal deterioration). They presented average diabetes duration of 9.33 years, resulting in 54.8% of the biopsies compatible with ND and, the remaining percentage, non-diabetic renal disease superimposed on ND18.
In Atlanta, USA, a kidney biopsy was performed on a population of 32 patients, African Americans, type 2 diabetics, with suspicion of NDRD (due to nephrotic, nephritic syndrome and rapid deterioration of renal function). Diabetic nephropathy was found in 41% of the patients, ND with coexisting glomerulonephritis in 38.7% and NDRD in 19.4% 19.
In Korea, a study with a population of type 2 diabetics (22 patients), with similar requirements for renal biopsy to previous work, detected ND in 36.4% of patients and NDRD in 63.6%, being the most important IgA nephropathy in 27% 20.
In France, after performing biopsy on 13 patients, due to microscopic hematuria or nephrotic syndrome, it was detected, in 61.5%, ND and, in the remaining 38.46%, another variety of NDRD, with 23% of nephropathy IgA and 30.77% of segmental focal glomerulosclerosis21.
In Spain, it was found that, in another group of 20 type 2 diabetic patients, undergoing renal biopsy, with absence of retinopathy, presence of hematuria, nephrotic syndrome and renal failure of unclear origin, 45% had ND, 35% nephropathy membranous, 15% vasculitis and 5% IgA nephropathy22.
There is another series of studies that have focused on NDRD, but with a population of basically macro-albuminuric patients, or with nephrotic syndrome, in which the predominant finding was focal segmental glomerulosclerosis and ND23-25. In other situations, diabetic patients with rapidly progressive renal deterioration have been analyzed, highlighting, in them, post-infectious glomerulonephritis, rapidly progressive glomerulonephritis and acute interstitial nephritis26,27.
There are few studies that have been centralized in a population similar to the one we studied: mainly diabetic elderly, with a low glomerular filtration rate and no macroalbuminuria. Ekinci et al., in 2013, report for the first time the results of renal biopsies performed on 31 type 2 diabetic patients, with reduction in GFR, 8 normoalbuminurics, and 6 microalbuminuric drugs, which compare with 17 macroalbuminuric patients. In the micro and macroalbuminurics patients the ND predominated, but, in the normoalbuminurics, interstitial and vascular changes prevailed (these of the type of renal arteriosclerosis). This supports that aging; hypertension and intrarenal vascular disease are important factors in this group of patients28.
In 2014, Shimizu et al. they review renal biopsies performed on type 2 diabetics and select only those performed on normoalbuminuric diabetic patients with low GFR, classifying them according to the recommendations of Fioretto et al. 29 of 15 patients, they found 6 (40%) with typical lesions of ND, but 9 (60%) with Class III lesions of Fioretto, characterized by advanced tubulointerstitial damage, vascular lesions and global glomerulosclerosis30.
In our series of cases of patients undergoing renal biopsy, a similar percentage of isolated diabetic nephropathy and hypertensive nephrosclerosis was found (28%). It is noteworthy that 18% of patients presented findings that allowed them to be classified into a mixed, diabetic and hypertensive variety, or ND with superimposed vascular disease, similar to Fioretto's class III. Hypertensive nephrosclerosis (28%) and chronic interstitial nephritis (20%) represent very important groups. In the last diagnosis, we should not be surprised by its prevalence, considering the age of the patients who underwent the biopsy, since it is a mainly geriatric population and the presence of associated pathologies, which frequently leads to the consumption of NSAIDs and analgesics. In this regard, in the study by Chong et al. in patients with NDRA, interstitial nephritis was detected in 48% of patients, followed by glomerulonephritis (40%) and hypertensive kidney disease (10%). However, within the group of 110 diabetic patients who underwent a biopsy, the clinical characteristics of 45 of them included mainly proteinuria in the nephrotic range and hematuria31. Similar findings describe Yaqub et al. in 34 patients with NDRD, in whom interstitial nephritis was present in 32% of the cases32. Renal artery stenosis was detected in 8.96% of the total of patients, a figure lower than that reported by Ritchie et al., in which hypertensive diabetic patients detected renal artery stenosis in 20.83% of 24 as those who underwent digital subtraction angiography33. IgA nephropathy only accounted for 5% of the cases, which is against other reports in the literature. However, the inclusion of patients with nephrotic syndrome should be emphasized, which explains the prevalence detected from 18.6% to 34% due to IgA nephropathy, with similar percentages for focal segmental glomerulosclerosis34-36.
This study raises a call to the medical community, both specialists and general practitioners, involved in the care of patients with type 2 DM. The possibility of the existence of entities other than the DN in non-proteinuric diabetic patients should always be considered. Regardless of age, with elevated nitrogen levels as a cause of impaired renal function. The realization of a diagnostic approach, from less to more complex, and not delay the percutaneous renal biopsy will allow early identification of patients whose prognosis will be impacted and, therefore, different therapeutic alternatives37.
Ethical responsibilities Protection of people and animals
The authors declare that no experiments have been conducted on humans or animals for this research.
Right to privacy and informed consent
The authors declare that patient data does not appear in this article.
Contribution of the authors
Dr. Leidy Yoana Aristizabal Gomez: review of medical records in the RTS platform, creation of the database, bibliographic search, participation in analysis and writing of the final article.
Dr. César Augusto Restrepo Valencia: recruitment of patients from the RTS Manizales outpatient clinic during the last 8 years, review of the database, bibliographic search, thematic advisor, active participation in the analysis and correction of the final article.
Dr. José Vicente Aguirre Arango: epidemiological advisor, database reviewer and statistical analysis.











 texto en
texto en