Introduction
Chronic kidney disease (CKD) is a serious public health problem whose prevalence has been estimated between 8-16 % of the population worldwide1. The management of this con dition represents a high cost, and a high percentage of these patients end up in renal replacement therapy (dialysis or transplantation)2. Furthermore, the accelerated increase in the number of patients with diabetes has a great impact on the development of diabetic kidney disease (DKD), as it is one of the most frequent complications of both types of dia betes. In turn, it is the leading cause of end-stage renal disease (ESRD), responsible for almost 50 % of cases in first world countries3.
For this reason, intensive interventions on the metabolic control of the disease are justified, in order to minimize the risk of ESRD and alleviate the hea vy socioeconomic burden that this condition represents2-4.
DKD occurs in up to 40 % of people with type 1 or 2 diabetes. This not only represents the risk of progressing to ESRD, but it is also expressed as a significant increase in cardiovascular morbidity and mortality4,5. Therefore, it is of paramount importance to identify early patients at risk of renal disease and/or delay in their progression, if it already exists. The diagnosis has always been based on the recognition of chronic hyperglycemia; the presence of albuminuria together with deterioration in renal function, expressed by the estimated glomerular filtration rate (eGFR, represented by hyperfiltration, albuminuria and proteinuria phases); the presence of other microvascular conditions, especially diabetic retinopathy; and the absence of signs of another type of kidney disease4,6,7 (Figure 1). In summary, the genesis of DKD is a complex process that can be modified with adequate metabolic control, where good glycemic management is essential.
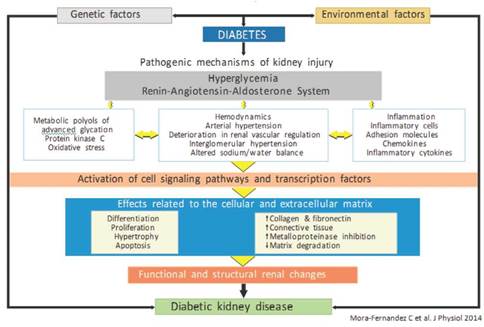
Figure 1 Factors involved in the genesis of diabetic kidney disease (modified by Mora-Fernández et al.7).
The use of metformin (in monotherapy or combined) has been consolidated as a first-line therapeu tic strategy8,9. This article reviews the role of metformin in DKD10, its risks and potential benefits.
Diabetes, kidney disease and metformin
For several decades, this biguanide has been considered as the antidiabetic medication par excellence. However, the acceptance of this drug was not easy. In Europe, it came with the publication of the United Kingdom Prevention Diabetes Study (UKPDS) in the 1970s11. Subsequent revisions by the ad hoc committee of the Federal Drug Administration (FDA) recommended that it be administered according to the eGFR, but never if it was less than 30 mL/min/1.73 m2, in an opinion that modified the appreciation of the safety of the drug, as discussed later12. A few weeks earlier, the European Medicines Agency (EAM) had adopted a similar position13.
As an original objective, metformin suppresses the hepatic production of glucose, it being its main mechanism of action to improve hyperglycemia in type 2 diabetes mellitus (DM2). Biochemically, metformin suppresses gluconeogenesis and stimulates glycolysis, in addition to inhibiting glycogenolysis, a pathway that contributes critically to elevate hepatic glucose production. Apart from these beneficial effects on hyperglycemia, metformin also improves insulin resistance and corrects dyslipidemia in patients with DM2 and various pleiotropic actions. Its anti-inflammatory properties have not yet been completely clarified10,14,15.
Is metformin truly nephroprotective?
It is well known that hyperglycemia is the main trigger of DKD, which initiates numerous microscopic and ultramicroscopic changes in the renal architecture16. The former includes thickening of the basal, glomerular and tubular membrane, as well as proliferation of the mesangium, arteriosclerosis, and abnormalities in the tubular glomerular junction. Ultramicroscopic changes are based on the podocyte injury ("effacement", loss of slit diaphragms and decrease of their density) and are considered as the core point in the genesis of proteinuria in the DKD17. Of the various mechanisms involved in inflicting damage to podocytes, the sustained aggression due to the increase in oxidative stress stands out16,18. Some of the different variables related to this higher production of reactive oxygen species include the reduction of glutathione, induced by hyperglycemia and the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase via hyperglycemia, advanced glycation end products (AGEs), protein kinase C (PKC), and renin angiotensin aldosterone system (RAAS). Otherwise, oxidative stress is a strong inducer of renal cell apoptosis, especially of podocytes16,19.
Given this complex scenario with many leading actors, it is desirable that antihyperglycemic drugs exercise some control, or correction, on several of these actors in order to delay the progress of DKD. However, only encouraging and consistent results with an effect on metabolic control have been obtained with new drugs, such as glyphzines or incretins (specifically, the agonists of the peptide-1 receptor, similar to glucagon [GLP-1], since there is no relevant information for inhibitors of dipeptidyl peptidase [DPP-4]) and inhibitors of sodium-glucose cotransporter-2 (SGLT2) 20,21.
As for metformin, a drug that has passed the test of time with 60 years of use, only some indirect data are known to point to protection. These should be considered from the experimental and clinical point of view, as summarized below.
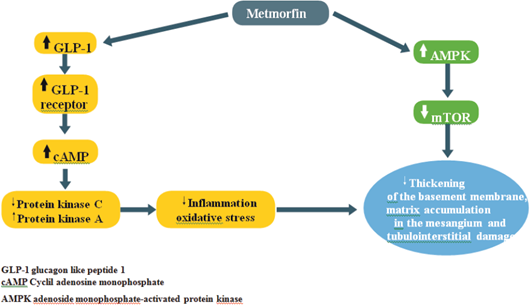
a) Experimentally, as already mentioned, hyperglycemia increases the production of reactive oxygen species (ROS) in kidney cells and, until recently, no drug had been shown to have the ability to prevent this situation. In 2010, however, Piwkowska and his group demonstrated that the activation of AMP-activated protein kinase (AMPK)22, generated by metformin, decreases the oxidation of the NADPH system. This leads to a decrease in the production of ROS in cultured human kidney podocytes.
AMPK adenoside monophosphate-activated protein kinase
While this activation of AMPK is the main therapeutic pathway of the effects of metformin, renal cells under conditions of hyperglycemia or proteinuria exhibit inactivation of cellular defense mechanisms; for example, AMPK and autophagy with activation of pathological pathways, such as rapamycin targets in mammals (mTOR), endoplasmic reticulum stress, epithelium-mesenchyme transition, oxidative stress, and hypoxia (Figure 2)23.
Another important aspect is the relationship between metformin and the GLP-1 concentration, a proven effect in both mice and humans24,25. The possible explanations are increase in the secretion of GLP-1, from the L cells of the intestine, and, to a lesser extent, reduction of its degradation by the enzyme DPP4. Another pathway mentioned is indirect, through the "pool" of bile acids26, which would act on their receptors of the L cells to favor the secretion of GLP-1. So far, the extent of such an effect is little known in patients with type 2 diabetes. Another study, in rats with diabetic nephropathy induced by streptozotocin, showed that the treatment of renal disease with metformin normalized all the biochemical changes and the energy deposits in the renal tissue28. At the transcriptional level, the supply of metformin caused a significant restoration in the values of messenger ribonucleic acid (mRNA), induced by oxidative stress, while inhibition occurred in proinflammatory genes, such as tumor necrosis factor alpha and interleukin-618.
In fact, some of these actions have already been proven, as in the following cases:
Ischemia and reperfusion, possibly due to the activation of AMPK and nitric oxide synthase29.
Restoration of podocytes in diabetic rats treated with metformin, perhaps by buffering or repressing oxidative aggression17, which would explain the reduction of albuminuria in patients with DM230.
Reprogramming of autophagy, an essential mechanism by which the cell degrades and recycles macromolecules and organelles. It works as an essential mechanism to maintain the homeostasis of the glomerulus and the renal tubule31, a process that is altered by oxidative stress and hyperglycemic load. The activation of AMPK by metformin or resveratrol duly restores the autophagy process32.
b) Regarding clinical support, several publications, derived from observational or other studies, highlight a certain role of nephroprotection, as summarized below.
In the publication of the Swedish Diabetes Registry, metformin was well tolerated in patients with CKD-3 and its use was associated with a 13 % reduction in total mortality21. Moreover, in comparison with sulfonylureas, the use of metformin is associated with a lower probability of deterioration of renal function33.
Crowley et al. 34, in a systematic review of several studies that used metformin in patients with historical contraindications (DKD with GFR of less than 60 mL/min/1.73 m2, heart failure or liver disease), found that its use was associated with reduction in total mortality and less readmissions due to heart failure, in patients with CKD or heart failure.
In the series of patients with acute myocardial infarction who will undergo emergency percutaneous coronary intervention (PCI), presented by Zeller et al. 35, there were fewer cases of ARF in the group receiving metformin than in the group that did not receive it. In a reduced number of carriers of concomitant CKD, the occurrence of ARF was similar with or without metformin, with no cases of lactic acidosis detected. These results corroborate those of Posma et al. 36, who administered metformin for four months to non-diabetic subjects who underwent PCI. In the treated group, there was less deterioration in the GFR and lower incidence of ARF secondary to the contrast, although neither of the two results was statistically significant.
Metformin and restriction of renal function
For several decades, one of the most important limitations imposed by regulatory authorities on metformin was renal function, establishing the limit of creatinine at 1.4 mg/dL for women and 1.5 mg/dL for men to contraindicate its use. This restriction remained, even though, already in 2009, both the ADA (American Diabetes Association) and the EASD (European Association for the Study of Diabetes) 37 postulated that metformin seemed safe, unless the eGFR fell below 30 mL/min/1.73 m2. In subsequent years, clinical studies and reviews were published that justified extending the use of metformin to patients with CKD-3 (i.e., between 59 and 30 ml/min/1.73 m2) 37-41, because these patients, with higher cardiovascular risk per se, benefited from this drug.
These actions culminated in the decision of the FDA12 and the EMA13 to allow the use of metformin including the group of CKD-3 patients, under certain conditions described below.
Metformin Associated Lactic Acidosis (MALA). Metformin is eliminated, mainly without being metabolized, by the kidney, glomerular filtration and tubular secretion. Therefore, patients with renal failure are more susceptible to its accumulation and development of lactic acidosis, a complication that can be fatal. In fact, the precipitating factor for lactic acidosis in those receiving metformin is an abrupt loss of tubular secretion. Such loss does not occur in stable CKD but is a characteristic feature of acute renal injury or rapid depletion of volume, associated with an intercurrent disease. Therefore, patients with CKD should be advised of the safety profile of the drug, so that they can be alert to potential adverse effects and provide written information about the suspension of metformin, if they experience any intercurrent illness that can lead to a rapid depletion of volume42.
Until recently, the relationship between lactic acidosis and metformin accumulation was poorly founded. This contributed to the regulatory warnings that, unfortunately, restricted its use, creating an exaggerated fear of the risk of such an adverse event43,44.
To date, it is well documented that the risk of MALA is very low (5/100,000 patients/year) and is usually associated with situations of poor general condition39,40,45. In addition, in recent years, the experience in the use of metformin has increased considerably, as revealed by Inzucchi et al.39 in their analysis of publications related to the topic between January 1950 and June 2014. They showed that lactate levels were frequently normal in patients receiving metformin with mild CKD (eGFR 60-90 mL/min) or moderate (30-60 mL/min). In studies in which the use of metformin was associated with increased lactate concentration, it did not rise to the level of evident lactic acidosis (defined as lactate level > 5 mmol/L and pH < 7.35). In reported cases of lactic acidosis, there were other associated causative factors, such as infection, liver failure, acute renal failure, or cardiovascular collapse. Clearly, the use of metformin was concomitant, but not causative, and the frequency of lactic acidosis, in patients under treatment with metformin, was the same as the one observed in patients with DM2 who did not receive metformin39,45.
Another relevant study, already mentioned, was the one derived from the analysis of a Swedish National Registry database, in 51,675 patients. It does not show an increase in risk of MALA in none of the groups, including carriers of CKD33. On the contrary, the authors report that its use was associated with a reduction in the risk of cardiovascular disease, severe infection and mortality from all causes, compared to treatment with insulin and reduction of total mortality, in reference to the other hypoglycaemic agents.
In the Cochrane review of Salpeter et al. 46 in 2010, the risk of lactic acidosis, fatal and non-fatal, was examined with metformin in DM2. It was concluded that, to date, there is no evidence that its use is associated with an increased risk of lactic acidosis, when prescribed under the conditions of the study. The authors state that 153 (53 %) of the 334 prospective studies allowed the inclusion of patients with renal failure, which represented a follow-up of 37,360 patients - years of metformin use. One of the included studies questioned standard contraindications, analyzing 393 patients with at least one contraindication for its administration and not finding any case of lactic acidosis in four years of follow-up47.
However, it is important to mention that the conjunction of small modifications in hydration, renal function, plasma concentration of metformin, or tissue oxygenation may trigger MALA48.
Consequently, given the rarity of lactic acidosis in the context of metformin treatment, a clinical study that could demonstrate the noninferiority of metformin compared with other drugs is totally impractical. Thus, records and observational studies are a reasonable alternative.
The recent review of the FDA12, considering the benefits and safety in patients with mild to moderate renal failure, regulated the use of the drug as follows:
Before initiating a treatment with metformin, calculate the GFR, using this parameter instead of the concentration of serum creatinine, since the GFR provides a better estimate of the renal function, considering important additional variables, such as age, sex, race or weight.
Contraindicated use if GFR is < 30 mL/min/1.73 m2.
Do not start metformin if GFR is between 30-45 mL/min/1.73 m2.
Measure the GFR, at least once a year, of all patients receiving metformin. In those at higher risk of developing renal failure (elderly, frail patients), monitoring should be more frequent.
If, during the treatment, the filtrate falls below 45 mL/min/1.73 m2, assess the risk/benefit ratio of continuing with metformin. If the filtrate drops below 30 mL/min/1.73 m2, it should be discontinued.
Stop treatment with metformin before a study with iodine contrast, in patients with filtrates between 30-60 mL/min/1.73 m2 and in patients with liver disease, alcoholism or heart failure. Re-assess the filtrate after 48 hours of the study and, if the renal function is stable, restart the treatment.
Additionally, the European Medicines Agency adds the following considerations13:
Before and during treatment, risk factors for lactic acidosis should be checked. Dehydration means a higher risk.
In fixed-dose combinations containing metformin, recommendations for dose adjustment of the other drug in patients with reduced renal function should be considered. It is possible that the dose adjustment can only be made using individual tablets of metformin and the other medication.
Some fixed-dose combination products are not recommended in patients with reduced renal function, because the other active ingredient in the combination should not be used. For example, dapagliflozin/metformin is not recommended in patients with a GFR < 60 mL/min; canagliflozin/ metformin and empagliflozin/metformin are not recommended in patients with a GFR < 45 mL/ min and should not be initiated in patients with a GFR < 60 mL/min.
Another important aspect is the strict control of renal function, when there is a change in the dose or class of antihypertensive that potentially affects the renal perfusion pressure (especially diuretics and ARS inhibitors). Adjust the dose of metformin as appropriate49.
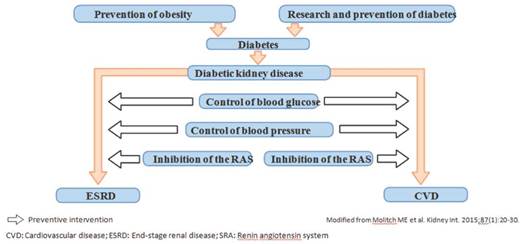
The best approach to avoid DKD is the prevention of diabetes itself. However, once diagnosed, glycemic control can delay the development of kidney disease. In those patients with DKD, strict and intensive control of blood glucose, blood pressure with RAS inhibitors and blood lipids is required to reduce vascular aggression and the risk of atherosclerotic cardiovascular disease8,9,37,42 (Figure 3).
Key messages
The risk/benefit balance of metformin is clearly favorable in most diabetic patients, especially when the changes in lifestyle are fully met. However, its use is not free of risks that both the doctor, the patient, and family members should know50. Under the current conceptualization of the drug, it is convenient to consider:
Its onset in patients with stage 4-5 CKD is not allowed and neither is supported by the evidence.
The patient must be informed that, like any other drug, metformin has advantages, disadvantages, and putative effects.
The risk factors identified for MALA include: acute renal failure, dehydration, hypoxemia, sepsis, alcohol abuse, liver failure, and shock.
Moreover, it should be noted that, in advanced age, patients with hemodynamic fragility, carriers with various comorbidities and CKD are at greater risk of MALA, despite the dose adjustment of metformin39,45,48,51.
Occasionally, a radiological study with contrast can lead to acute renal failure (ARF), which is why in patients with eGFR <45 mL/min the suspending metformin 48 hours before the study and restarting it 48 hours later is recommended. So far, contraindications for use in patients with liver damage and in pregnant women are maintained, with the limitations imposed in cases of severe deterioration of renal function.
Protection of people and animals
The authors state that no human or animal experiments have been performed for this research.











 texto en
texto en