Introduction
Posttransplant lymphoproliferative disease (PTLD) represents one of the most severe complications in hematopoietic cell and solid organ transplant (SOT). The risk of occurrence is 10-20 times higher than in the general population1,2. It affects 1-20 % of patients with SOT2,3. Incidence is 0.7-2.9 % in kidney transplant1,3, being higher in intestine, pancreas and lung transplantation (4-7 %)1,3. It is characterized by the uncontrolled production of B lymphocytes and, less frequently, T lymphocytes3,4. There are associated risk factors, such as Caucasian race, primary infection with the Epstein Barr virus (EBV), use of antilymphocyte antibodies in induction therapy, coinfection with cytomegalo-virus (CMV), antiviral therapy, graft rejection and previous dialysis2,5. PTLD during the first year of transplantation, or early occurrence, is the most frequent form (up to 40 %) and can be associated with positive CD20. It originates in B cells, where the role of the virus is uncertain6. The late form, or monomorphic type, appears after the first year of transplantation, is EBV negative, originates in T and natural killer cells, and has a worse prognosis7. Differentiating PTLD from episodes of rejection or viral infection is difficult when the tumor infiltrates the graft because the clinical and histopathological findings are very similar. It is more frequent in children with aggressive immunosuppression who have not had infection with EBV8.
The lymphoproliferative alterations are classified into benign hyperplasia, where there is diffuse proliferation of mononuclear cells with preserved tissue; polymorphic type PTLD, characterized by lymphocytes in different phases of differentiation, tissue damage and areas of necrosis; monomorphic type, where the tissue has neoplastic transformation; and, finally, classical Hodgkin's lymphoma7,9,10. PTLD arising from B cells is identified with CD20 staining. The reduction of immunosuppression is the first line of treatment in early polymorphic PTLD9.
Remission after treatment with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy reaches 75 %, and is higher when radiotherapy or tumor removal is performed. Mortality is 40-70 % at 5 years8,9.
We are reporting the case of a patient with a mass in the transplanted kidney compatible with CD20-positive and EBV-positive polymorphic PTLD, who received treatment with R-CHOP, showing good response and favorable clinical evolution.
Description of the clinical case
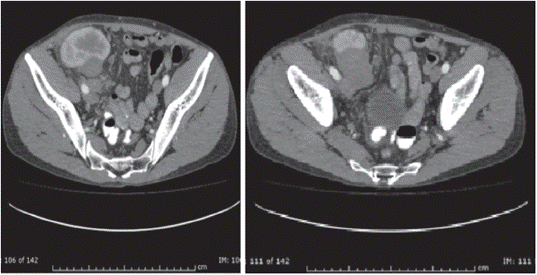
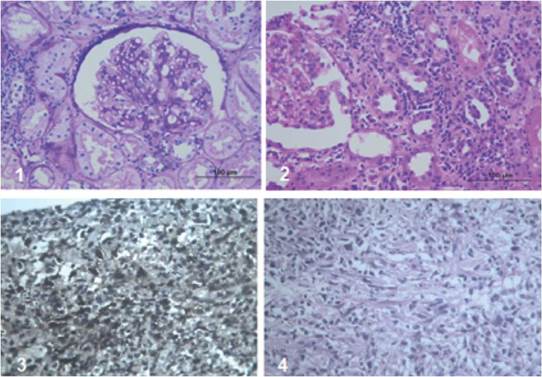
The patient is a 38-year-old man with a diagnosis of chronic kidney disease (CKD) secondary to IgM glomerulonephritis, on hemodialysis for 9 years. He received a transplant from a deceased donor in January 2008, intermediate risk for CMV, IgG serology for donor/recipient EBV positive. He received induction with basiliximab and maintenance therapy with cyclosporine, maintaining serum C2 levels as recommended in those years, as well as mycophenolate mofetil 2,000 mg a day and prednisolone 5 mg a day. The average creatinine during the first year was 1.3 mg/dL. Three months after transplantation, he exhibited proteinuria of 1.2 g/day. Thus, he underwent renal biopsy, finding acute tubular necrosis, without evidence of other alterations. However, proteinuria persisted, and a new renal biopsy was performed, finding transplant glomerulopathy and plasmacytic infiltrate, without viral inclusions or acute cellular or humoral rejection; immunosuppression was optimized, and follow-up was continued. Fifteen months post-transplant, he presented abdominal and graft pain; Doppler ultrasound of the graft showed a hypoechoic, heterogeneous mass with irregular morphology of well-defined contours (49.7 x 37 x 33.3 mm) with some vascular structures in its interior. At the moment, he had proteinuria of 9 gr/24 hours. The study was completed with abdominal scan (Figure 1) that confirmed the finding of a hypodense mass of 40 x 48 x 40 mm, dependent on the lower pole of the renal graft. A biopsy of the lesion was performed, showing an interstitial mononuclear inflammatory infiltrate that permeated some renal tubules with abundant eosinophils, and, in other fragments, tumor lesion composed of plasma cells, lymphocytes with prominent nucleoli, and few macrophages. Immunohistochemistry showed abundant CD20-positive B cells, intermixed with CD3-positive T lymphocytes, CD30-positive and some EBV- positive activated immunoblasts. This was associated with chronic interstitial tubule nephritis and nephropathy mediated by incipient immune complexes. Plasmocytes with polyclonal expression and light kappa and lambda chains were identified, findings compatible with a diagnosis of EBV-positive polymorphic PTLD, (Figure 2).
Renal graft in the right iliac fossa, normal size, 50x48x40 mm hypodense mass of lobulated contour, located in front of the iliac vessels, dependent on the lower pole of this kidney, compatible with neoplastic lesion of the renal graft.
Immunoperoxidase stains in the tumor lesion
Plasmocytes with polyclonal expression, light Kappa and Lambda chains.
CD20-positive B cells.
CD3-positive T lymphocytes.
CD30-positive and EBV-positive activated immunoblasts.
Initial treatment consisted in decreasing immunosuppression, interrupting ciclosporin, and starting sirolimus. Mycophenolate mofetil was reduced to 1,000 mg/day and subsequently to 500 mg/day. He was assessed by hematology, which started rituximab 375 mg/m2 weekly, four doses. The control images showed a decrease in the mass by only 20 %, so CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone) treatment was given for 6 cycles.
Evolution of the patient was favorable. Proteinuria and dyslipidemia persisted, with no haematuria and stable renal function. He adequately tolerated chemotherapy, did not require radiotherapy, nor nephrectomy of the transplant and, after treatment, the mass decreased significantly until disappearing at one year of follow-up. The patient progressed satisfactorily, with disappearance of the mass, creatinine of 1.0 mg/dL and proteinuria lower than 500 mg/day. The SCAN PET report, at the end of the treatment, was normal.
Discussion
The case presented shows early EBV positive PTLD, with intermediate risk for CMV infection.
Two biopsies of the transplanted kidney were performed due to proteinuria and hematuria, which were not compatible with malignancy. The second biopsy showed severe interstitial plasmocytic infiltration; therefore, studies were requested for infection with polyomaviruses, which were negative. He received universal prophylaxis with valganciclovir for CMV for 3 months.
A risk factor clearly related to PTLD is the degree of immunosuppression. However, the patient had a low immunological risk, basiliximab was used for induction and the most frequent association is thymoglobulin, which the patient never received. The ciclosporin levels were between 900 and 1,300 ng/ mL during the first 3 months, which does not correlate with deep immunosuppression.
As published in the literature, the treatment of PTLD must be individualized and depends on the aggressiveness of the tumor, histological changes, the serological status of EBV, and the location of the tumor lesion. All these aspects were considered when deciding upon the treatment scheme that would be indicated to the patient.
The decrease in immunosuppression is the first line of treatment in patients with less aggressive polymorphic PTLD, due to the good prognosis9. The literature has documented that, even in less aggressive cases, the reduction of immunosuppression may be insufficient4. In this patient, the response to reduced immunosuppression was inadequate; the positivity of histological stains for CD3, CD20, CD30 and EBV justified the addition of rituximab, without reaching a decrease in the mass either, so CHOP chemotherapy was added. There is no clear recommendation regarding the joint management of rituximab and CHOP, but the truth is that it can be administered concomitantly or sequentially with similar results11.
The finding of atypical lymphoid cells supports the diagnosis of PTLD. However, the histological findings are very varied, and it is very difficult to differentiate PTLD from infection, rejection or hyper-sensitivity associated with drugs9.
Sometimes, it is necessary to perform radiotherapy or surgery1. The patient did not present a mass effect, nor severe compromise of renal function, so these interventions were not required. On the other hand, there seems to be a relationship between the appearance of PTLD and immunosuppression with tacrolimus. The patient was under treatment with cyclosporine and, upon diagnosis of PTLD, immunosuppression was changed to sirolimus despite proteinuria, and 25 % of the dose of mycophenolate mofetil was maintained as recommended by many studies11,12. The literature is unclear on how much to reduce the dose of immunosuppressants; critically ill patients should receive minimal doses or even be treated free of immunosuppression and maintain prednisolone at a dose of 7.5 to 10 mg/day13. In less severe cases, the dose of the calcineurin inhibitor could be reduced to 50 %, and two weeks later another reduction of 50 % can be made, while mycophenolate or azathioprine can be suspended14. Antiviral therapy is recommended by many authors in children with PTLD; its usefulness in adults is controversial13.
Rituximab is a chimeric anti-CD20 monoclonal antibody with potent B cell depleting activity. It was initially used for the treatment of follicular lymphoma. It has been effective in the treatment of PTLD, has improved survival, and is considered a second-line drug in PTLD. The dose is 375 mg/m2/ weekly, for four doses, and monotherapy is used in SOT receptors that do not respond to the reduction of immunosuppression in the absence of circulating CD2012. Rituximab does not restore the specific immunity of the T cell against EBV, which explains why PTLD can recur at 4-8 months of follow-up15. Rituximab has been used with sirolimus to improve patient and graft survival15.
Tsai DE and colleagues analyzed 42 patients with PTLD. The favorable response in reducing immunosuppression was determined by the normal value of LDH and the viral load with respect to the baseline. The overall response rate was 44 % and the one-year survival rate was 67 %, the relapse rate was 21 % and the rejection rate was 22 %16. CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) based chemotherapy has shown remission of PTLD in 75 % of cases17. Penn conducted a retrospective study in Israel, which showed that 5-year survival is less than 5 % in patients treated with monochemotherapy, unlike treatments such as CHOP and Pro MACE - CytaBOM (cyclophosphamide, doxorubicin, etoposide, prednisone, bleomycin, cytarabine, vincristine, methotrexate and leucovorin), showing remission close to 75 %17.
Radiation therapy and/or surgery is effective when the PTLD causes mass effect, pain or bleeding, or when the graft is affected by the tumor, in which case additional systemic treatment is required1,17.
The response was adequate, with no deterioration of renal function and a decrease in the size of the mass. Some studies have found an increase in episodes of rejection by reducing immunosuppression; the patient did not have this complication. The possibility of relapse is variable, close to 20%, so strict monitoring of these patients should be continued. In high-risk patients it seems to be useful to monitor the viral load for EBV.
Conclusion
The appearance of PTLD in the renal graft is rare, being more frequent in the early-onset polymorphic type. Tumor involvement at the graft level is infrequent. Different reports in the literature have shown an incidence of up to 2.9 % in patients with kidney transplantation, without precise statistics of tumor involvement in the graft due to PTLD. The primary objective of treatment is to cure the disease and concomitantly preserve the function of the graft. Our patient responded satisfactorily to the rituximab scheme together with CHOP, thus preserving the renal graft, without altering the renal function or requiring nephrectomy.











 texto en
texto en