Introduction
Tn Colombia, there have been programs of kidney transplant for 40 years as a definitive alternative in the treatment of the terminal chronic kidney disease (TCKD). In 2015, 979,409 individuals with TCKD were identified in Colombia, 34,469 of them were under renal replacement therapy (RRT).1 According to the latest report from the National Information System on Donation and Transplants of the National Institute of Health,2in 2015, 1,906 persons were on the waiting list for a kidney transplant. The average wait time was 400 days and 61 patients died on the waiting list. 844 kidney transplants were carried out.2 According to our knowledge, in Colombia there are no data on the verall survival in transplanted patients. However, one study showed a long-term survival of 90.7% for the patients and 90.4% for the grafts.3
Kidney transplantation is the definitive treatment of choice for patients with TCKD, with an ostensible lower mortality with respect to patients on RRT with dialysis.4 In order to successfully achieve graft survival, pharmacological immunosuppression regimens that allow to achieve acceptance of the transplanted organ must be followed.5 The introduction of calcineurin inhibitors (CNIs), one of the main drugs used for post-transplant immunosuppression, significantly improved the survival of transplanted patients, reaching a survival of more than 90%.
However, it has been evidenced in the long term a direct relationship between the time of exposure/dose of the drug and the development of adverse events.The cause of deterioration of graft function is multifactorial. In contrast to what was previously thought, the majority of cases of renal graft loss have an identifiable alloimmune cause,6 and are also related to the deleterious effects of the CNIs on patient and graft survival. The CNIs generate multiple adverse effects, among which are nephrotoxicity, hypertension, hyperlipidemia, de novo diabetes mellitus, neoplasm and viral infection.7,8,9
With the advent of new immunosuppressive therapies, it has been possible to design therapeutic strategies aimed to minimize the negative impact of calcineurin inhibitors on chronic graft dysfunction, and to reduce renal, cardiovascular and infectious comorbidities.10,11 The rapamycin inhibitor drugs or mTOR (Mammalian Target of Rapamycin) inhibitors are immunosuppressive agents with antiproliferative and antimigratory capacities. This confers them a potential role to protect against kidney graft dysfunction, reduce acute rejection, optimize the cardiovascular profile and reduce the appearance of de novo tumors.11,12 All this can, theoretically, preserve the renal function and the life in the long-term in a selected group of patients. However, the side effects could counteract these benefits in a longer term.13,14,15 Thus, the study aims to evaluate the experience of our institution with the switch of an anticalcineurinic agent to anmTOR inhibitor in 5 years of follow-up, in order to evaluate the graft survival, adverse effects and complications with the use of these drugs.
Materials and methods
It was conducted an observational retrospective study which included patients with TCKDwho were transplanted in the Valle de Lili Foundation from October 1995 until December 2013. Since the introduction of mTOR inhibitors in the country, these patients received an indication for suspension of CNI after the third month post-transplant.
All patients underwent renal biopsy prior to the switch. No patient was diagnosed with chronic nephropathy, IFTA >40 % or proteinuria >350 mg/24h. Patients coming from other cities were excluded, because for them, the post-transplant follow-up was shorter than 30 days. The use of mTOR inhibitors was indicated in patients with a history of cancer, de novo neoplasia, nephrotoxicity documented by biopsy and neurotoxicity.
The data of the recipients were obtained from the institutional registry of renal transplantation TRENAL), created since 2009. The collection of data specific for the study was carried out in an alternate database, through the review of clinical records and paraclinical reports.
The CNI toxicity, both renal and neurological, was established by biopsy and brain magnetic resonance imaging, respectively. For the analysis of the adverse effects and complications, were taken the definitions of the KDIGO 2009 guidelines,16 which defined proteinuria as a urinary excretion or total proteins greater than 300 mg/day; dyslipidemia as a register of triglycerides >500 mg/dL and/or LDL >100 mg/dL; anemia as a hemoglobin level <13.5 g/dL in men and<12 g/dL in women. In addition, a diagnosis of diabetes was made according to the ADA guidelines.17 The Banff 2007 criteria and classification18 were used to define the acute graft rejection.
A descriptive statistical analysis was conducted for all variables and for selected subgroups using STATA 12.0. The Shapiro-Wilk test was used to determine the normality of the data. Those data with a normal distribution were summarized as mean ± standard deviation, otherwise they were summarized by medians and interquartile ranges. The qualitative variables were presented in proportions and analyzed using the chi-square test. The KaplanMeier method was used for the analysis of survival of the patient and the graft and for the study of the incidence of acute rejection, and the log-rank test was used to compare the different groups.
Results
Of 1273 kidney transplants, the switch from CNI to mTOR inhibitors was done in 166 (13%), of which 25 (15.1%) were under treatment for less than 6 months. The median age was 48 years (IQR = 35-57), 59% consisted of men and 81% of the transplants were from a deceased donor.
85 % (n=141) were under treatment with cyclosporine; and 15 % (n=25), with tacrolimus. 78% (n = 129) were switched to sirolimus and 22% (n = 37) to everolimus. The median post-transplant follow-up was 84 months (IQR =43-126); and the median duration of treatment with mTOR inhibitor of 37 months (IQR =10-71). Acute rejection occurred in 9.6 % (n=16), none of which was humoral; graft loss in 12.6 % (n=21); and death in 4.2 % (n=7). The causes of death were malignancy (n=4) and sepsis (n=3) (Table 1).
Table 1 Demographic and clinical characteristics of kidney transplant patients switched to mTOR inhibitors
| Age (years)* | 48 (35-57) |
| Type of donor | n (%) |
| Living | 31 (19%) |
| Deceased | 135 (81 %) |
| Gender | n (%) |
| Female | 68(41%) |
| Male | 98 (59 %) |
| CNI | n (%) |
| Cyclosporine | 141 (85 %) |
| Tacrolimus | 25 (15 %) |
| mTOR inhibitor | n (%) |
| Sirolimus | 129 (78 %) |
| Everolimus | 37(22 %) |
| Time of post-transplant follow-up (months)* | 84 (43-126) |
| Time of treatment with mTOR inhibitor (months)* | 37 (10-71) |
| Acute rejection, n (%) | 16 (9,6 %) |
| Graft loss, n (%) | 21 (12,6 %) |
| Death, n (%) | 7 (4,2 %) |
*Median (IQR)
IQR: interquartile range; CNI: calcineurin inhibitors; mTOR inhibitors: rapamycin inhibitors.
In 56.6 % (n=94) of the patients, the cause of switch to mTOR inhibitor was the CNI toxicity. This cause was followed by malignancy, in 27.1% (n = 45), and by infection with polyomavirus 4.8% (n = 8). 53% (n = 24) of the patients with malignancy had skin cancer; 17.8% (n = 8), post-transplant lymphoproliferative disorder (PTLD); and 28.9% (n = 13), other tumors. Of these patients, 6 (13%) had a relapse of malignancy.
In 62 patients (37 %), the mTOR inhibitor was withdrawn, and the main causes for this were: de novo pathological proteinuria (48.8 %, n=30), late acute rejection (19.4 %, n=12) and adverse events associated withmTOR inhibitors (11.3 %, n=7), such as diarrhea and stomatitis, among others. In two patients, the cause of withdrawal of the mTOR inhibitor was the development of infections: one patient presented urinary infections with multiresistant Klebsiella pneumoniae and one patient had pulmonary tuberculosis (Table 2).
Table 2 Characteristics of the use of mTOR inhibitors
| Causes of switch to mTOR inhibitor | n (%) |
|---|---|
| CNI toxicity | 94 (56,6 %) |
| Malignancy | 45 (27,1 %) |
| Polyomavirus | 8 (4,8 %) |
| Chronic graft dysfunction | 5 (3 %) |
| Gastrointestinal adverse effects | 4 (2,4 %) |
| Other | 10 (6 %) |
| Post-switch complications | n (%) |
| Dyslipidemia | 94 (56,6 %) |
| Anemia | 34 (20,4 %) |
| Diabetes mellitus | 7 (5,4 %) |
| Acute myocardial infarction | 5 (3 %) |
| Hepatitis B and C | 2 (1,2 %) |
| Stroke | 1 (0,6 %) |
| Causes of withdrawal of the mTOR inhibitor, n(%) | n=62, (%) |
| Pathological proteinuria | 30 (48,4 %) |
| Late acute rejection | 12 (19,4 %) |
| Adverse effects of mTOR inhibitor | 7 (11,3 %) |
| Dyslipidemia | 5 (8,1 %) |
| Infection | 2 (3,2 %) |
| Myopathy | 1 (1,6 %) |
| Anemia | 1 (1,6 %) |
| Other | 4 (6,5 %) |
mTOR inhibitors: rapamycin inhibitors; CNI: calcineurin inhibitors.
The complications that arose after the switch to mTOR inhibitors were dyslipidemia in 56.6 % of cases (n=94), anemiain 20.4 % (n=34), de novo post-transplant diabetes mellitus in 5.4 % (n=7), acute myocardial infarction in 5.4 % (n=7), diagnosis of hepatitis B and C in 1.2 % (n=2),and stroke occurred in 1 patient (0.6 %). (Table 2).
Among the 141 patients under treatment with mTOR inhibitor for more than 6 months, it was observed that the median serum creatinine (Scr) at 6, 12, 24, 36, 48 and 60 months after the switch was: 1.49 mg/dL (IQR=1.1-2), 1.42 mg/dL (IQR =1- 1.9), 1.38 mg/dL (IQR =0.98-1.76), 1.3 mg/dL (IQR =0.91-1.69), 1.35 mg/dL (IQR =0.89-1.7) and 1.25 mg/dL (IQR =0.88-1.7), respectively.
The overall graft survival after the switch to mTOR inhibitor was analyzed. It was found that, at one year it was 96.6 %; and at five years, 83.5 %.
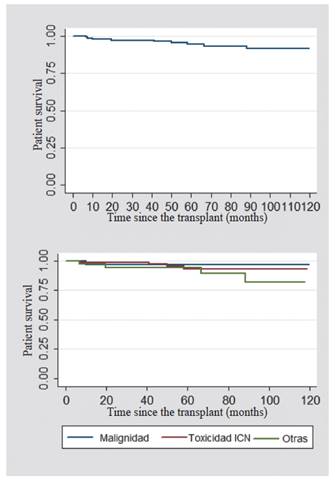
When comparing the causes for which the switch to mTOR inhibitor was made, the overall survival of the graft at the first and fifth year was 96.8% for the patients whose cause of switch was malignancy; 98.7% -80% for those who were modified due to a CNI toxicity; and 92% -77% for other causes (Figure 1). The overall survival of the patient after the switch to anmTOR inhibitor was also analyzed. In the first year, it was 98%; and at five years, 97%. In the patients whose cause of switch to the mTOR inhibitor was malignancy, it was found a survival of 96.8 %; for those who had CNI toxicity, it was 98.7 %; and for other causes, 97.3 % (Figure 2).
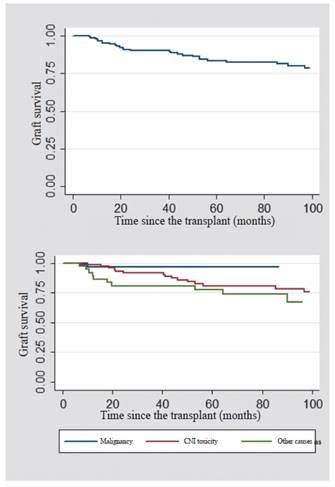
Figure 1 Renal graft survival (upper: patients switched to mTOR inhibitors; lower: according to the indications for switching to mTOR inhibitors)
Discussion
Until 2013, more than 1,500 patients were in post-transplant follow-up, most of them treated in the postoperative period with CNI as the first management option.14 .In our cohort, 12. 6% of patients required a change to mTOR inhibitor. The main cause of switching was CNI toxicity (mainly renal and neurological), demonstrated by renal biopsy and brain magnetic resonance imaging.The next cause was the diagnosis of malignancy, mostly skin cancer. These causes have been reported in other studies.15 In the majority of patients, a switch to sirolimus was carried out.
It is known that the early use of mTOR inhibitors is associated with an increase in graft rejection, infections at the surgical site and alteration of the healing process.19,20 .For this reason, in our study the switch to mTOR inhibitors was made in patients with more than 3 months post-transplant.
37% of the patients in our study required the withdrawal of the mTOR inhibitor. The main cause for this was pathological proteinuria and late acute rejection, despite the fact that our population had a good renal function prior to the switch, with glomerular filtration rates higher than 40mL/min/ M2 and with proteinuria below 500mg /24h. It has been demonstrated that mTOR inhibitors have high rates of adverse events and that these are the main cause of withdrawal of the drug.21
Of the 45 patients with malignancy, 13 % had a relapse after the switch to mTOR inhibitor. Skin cancer was the main neoplasm in our study. Hematological and solid organ neoplasms were also evidenced, to a lesser extent.22
It has been described that sirolimus is related to the development of dyslipidemia. In fact, we found that it was the main post-switch complication in our patients. In our study, the onset of anemia with requirement of erythropoietin treatment was frequent after the switch to mTOR inhibitors.23 De novo diabetes occurred in 5.4 % of our population.
The survival of the graft and the patient found is good and is not different from that reported in other studies.23,24 Acute graft rejection occurred in 9.6% of the patients. One study demonstrated that the incidence of acute rejection with the use of mTOR inhibitors varies between 10% and 20%.25
In numerous studies that show the benefit of the switch to mTOR inhibitors, the quality of the tests to justify the replacement of the CNI by the mTOR inhibitor is inconclusive.13,14 The switch to a mTOR inhibitor has very precise indications and must be carried out under specific conditions, with the intention of reducing the risks inherent to its use that may affect the survival of the patient and the graft.
In the population of kidney transplanted patients who were switched to mTOR inhibitors, we found a low incidence of relapse or onset of malignant disease. A large number of patients had adverse drug events. The main post-switch complications were proteinuria, dyslipidemia and anemia. In patients with specific indication for the use of mTOR inhibitors, the protocol with renal graft biopsy, glomerular filtration rate higher than 40ml/ min/M2 and proteinuria lower than 350mg/24h prior to the switch to mTOR inhibitor allowed for greater safety, less adverse effects and good graft and patient survival.
Ethical responsibilities
Protection of people and animals
The authors declare that no experiments were performed on human beings or animals for this research.
Data confidentiality
The authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consent
The authors state that patient data do not appear in this article.
Contribution of the authors
Liliana Mesa-Ramirez: design of the study, writing of the article, review and edition of the manuscript.
Juan C. Gómez-Vega, Jessica Pino-Escobar: design of the study, database, literature review, writing of the article.
Lina M Rivera: literature review, writing of the article.
Eliana Manzi-Tarapues: design of the study, data analysis, review and edition of the manuscript.
Juan G. Posada-Chávez, Johanna Schweineberg-López, Carlos E. Durán-Rebolledo, Jorge I. Villegas-Otálora, Oscar J. Serrano-Ardila, Luis A. Caicedo-Rusca: review and edition of the manuscript.











 texto en
texto en