Original research article
Evolution of glomerulopathies associated with rheumatoid arthritis
José Lucas Daza1
*
Yaroslad De La Cruz1
Cintia Marín1
Martín Zapata1
Fernando Segovia1
Luis José Daza1
Graciela De Rosa2
John Galindo3
1 Department of Nephrology of the Hospital de Clínicas, University of Buenos Aires, Argentina
2 Department of Pathology of the Hospital de Clínicas, University of Buenos Aires, Argentina
3 M.D, Pedagogical and Technological University of Colombia, Boyacá, Colombia
Abstract
Introduction:
Rheumatoid arthritis is one of the most common clinical syndromes within rheumatological conditions and its association with glomerular diseases is rare.
Objective:
To describe the histopathological findings in renal biopsies in patients with rheumatoid arthritis and to correlate them with the clinical and laboratory manifestations at the beginning, at 6 months and at one year of follow-up.
Patients and Methods:
This is a retrospective observational study conducted in the Hospital de Clinicas "Jose De San Martin" in Buenos Aires, Argentina; Where we included 41 patients diagnosed with RA (ACR 1987) in a period of 20 years. Histopathological diagnoses of membranous nephropathy (MN), minimal change disease (MCD), secondary amyloidosis (AA), focal and segmental glomerulosclerosis (FSGS); mesangial glomerulopathy (MGP) and glomerulonephritis with extracapillary proliferation (GNEC) were included. Histopathological description, different treatments, years of evolution of rheumatoid arthritis Clinical and laboratory characteristics were analyzed during the first 6 months and one year of follow-up in order to determine the progression of renal failure calculated through the formula of MDRD of 4 variables (Modification of diet in renal disease) and the increase of proteinuria.
Results:
The most frequent histological finding was amyloidosis with 34,1 % (n=14), followed by mesangial glomerulopathy 21,9 % (n=9), membranous nephropathy 19,5 % (n=8), glomerulonephritis with extracapillary proliferation 12,1 % (n=5), focal and segmental glomerulosclerosis 7,3 % (n=3) and minimal change disease 8,2 % (n=2). Nephrotic syndrome was the most frequent presentation in patients with amyloidosis in 85,7 %, microhematuria occurred in 100 % of patients with MPG and in 80 % of patients with GNEC. In patients with AA, moderate to severe interstitial fibrosis occurred in 85,7 %, followed by GNEC and NM with 80 % and 40 % respectively. The 24-hour proteinuria, creatinine and glomerular filtration rate estimated by MDRD at 6 months and 12 months were evaluated. Concluding, that patients with AA, FSGS and GNEC had greater progression of renal failure at 12 months; the opposite occurred in patients with minimal change disease (MCD) and mesangial glomerulopathy (MGP) who had a lower progression of renal failure at one year of follow-up; There was a correlation in the glomerulopathies that had greater deterioration of the renal function had greater interstitial tubule involvement as was the case of amyloidosis. The glomerulopathies that presented greater proteinuria at the beginning were membranous nephropathy, amyloidosis and minimal change disease. Both membranous nephropathy and minimal change disease had partial remission at one year, in contrast to amyloidosis, which showed progression of proteinuria at 12 months of follow-up.
Conclusion:
The glomerulopathies that presented greater progression of renal failure at 1 year based on the estimation by MDRD 4, had a higher renal tubular interstitial involvement in renal biopsy and these were amyloidosis (AA), segmental focal glomerulosclerosis (FSGS), glomerulonephritis with proliferation extracapillary On the other hand, those with the best evolution in relation to the degree of proteinuria and the glomerular filtration rate determined by the MDRD4 equation were mesangial glomerulopathy, minimal change disease, and membranous nephropathy.
Key words: Rheumatoid arthritis; glomerulopathies; urinary sediment; MDRD; renal failure
Resumen
Introducción:
La artritis reumatoidea (AR) es uno de los síndromes clínicos con mayor frecuencia dentro de las afecciones reumatológicas y su asociación con las enfermedades glomerulares es poco frecuente.
Objetivo:
Describir los hallazgos histopatológicos en las biopsias renales en pacientes con artritis reumatoidea y correlacionarlos con las manifestaciones clínicas y de laboratorio al inicio, a los 6 meses y al año de seguimiento.
Pacientes y métodos:
Es un estudio observacional retrospectivo realizado en un hospital Universitario en Buenos Aires, Argentina. Se incluyeron 41 pacientes con diagnóstico de artritis reumatoidea de acuerdo a los criterios establecidos por el Colegio Americano de Reumatología publicados en 1987; en un período de 20 años. Se incluyeron diagnósticos histopatológicos de nefropatía membranosa (NM), enfermedad de cambios mínimos (ECM), amiloidosis secundaria (AA), gloméruloesclerosis focal y segmentaria (GEFS); glomerulopatía mesangial (GPM) y glomerulonefritis con proliferación extracapilar (GNEC). Las características clínicas, de laboratorios, la descripción histopatológica, los años de evolución de la artritis reumatoidea y los diferentes tratamientos fueron analizados durante los primeros 6 meses y al año del seguimiento. Con esto, se buscó determinar la progresión de la insuficiencia renal, calculada a través de la fórmula de MDRD (Modification of Diet in Renal Disease) de 4 variables y el aumento de la proteinuria.
Resultados:
El hallazgo histológico más frecuente fue la amiloidosis, con un 34.1 % (n=14), seguido de la glomerulopatía mesangial (21,9 %, n=9), la nefropatía membranosa (19,5 %, n=8), la glomerulonefritis con proliferación extracapilar (12,1 %, n=5), la glomeruloesclerosis focal y segmentaria (7,3 %, n=3) y enfermedad de cambios mínimos (8,2 %, n=2). El síndrome nefrótico fue la forma de presentación más frecuente en los pacientes con amiloidosis (en un 85,7 % de los casos), la microhematuria se presentó en el 100 % de los pacientes con GPM y en el 80 % de los pacientes con GNEC. En el 85,7 % de los pacientes con AA, se presentó fibrosis intersticial moderada a severa, mientras que en la GNEC y la NM la fibrosis se observó en un 80 % y 40 % respectivamente. Se evaluó la proteinuria de 24 horas, la creatinina y la filtración glomerular estimada por MDRD a los 6 y a los 12 meses. Se concluyó que los pacientes con AA, GEFS y GNEC presentaron mayor progresión de la insuficiencia renal a los 12 meses. Lo contrario sucedió en los pacientes con enfermedad de cambios mínimos (ECM) y glomerulopatía mesangial (GPM), los cuales tenían una menor progresión de la insuficiencia renal al año de seguimiento. Hubo una correlación entre las glomerulopatías que tenían mayor deterioro de la función renal en las cuales se observó a su vez, mayor compromiso tubulointersti-cial, (este fue el caso de la amiloidosis). Las glomerulopatías que presentaban mayor proteinuria al inicio eran la nefropatía membranosa, la amiloidosis y la enfermedad de cambios mínimos. Tanto la nefropatía membranosa como la enfermedad de cambios mínimos, tenía remisión parcial tras un año, a diferencia de la amiloidosis, la cual presentaba progresión de la proteinuria a los 12 meses de seguimiento.
Conclusión:
Las glomerulopatías que presentaron mayor progresión de la insuficiencia renal al año, con base en la estimación por MDRD4, tenían en la biopsia renal mayor compromiso tubulointersticial. Estas fueron la amiloidosis secundaria, la glomeruloesclerosis focal y segmentaria, y glomerulonefritis con proliferación extracapilar. Por el contrario, las de mejor evolución respecto al grado de proteinuria y tasa de filtrado glomerular determinado por MDRD4, fueron la glomerulopatía mesangial, la enfermedad de cambios mínimos y la nefropatía membranosa.
Palabras clave: artritis reumatoidea; glomerulopatías; sedimento urinario; MDRD; insuficiencia renal
Introduction
Rheumatological diseases such as systemic lupus erythematosus and systemic sclerosis have a direct impact on kidney problems. Rheumatoid arthritis (RA) is one of the most frequent clinical syndromes within the rheumatological affections and its association with glomerular diseases is not well known. Renal manifestations in RA are very varied and may be due to the disease itself or to the drugs used for its treatment.1 RA has been associated with a wide variety or renal disorders, due to its chronic inflammatory component, the exposure to drugs or the toxicity thereof.2
In a study conducted by Yoshinaga et al., on 154 ambulatory patients with RA, it was found that urinary alterations such as proteinuria and microhematuria were present in one third of cases. In addition, the indicators of tubular damage were high both in histology and in urinary levels of beta-2 microglobulin and N-acetyl-beta-D-glucosaminidase.3 The main glomerulopathy was amyloidosis, which had greater tubular commitment and worse evolution.
Mesangial glomerulopathy and membranous nephropathy derived from treatment with gold salts and penicillamine have been described as the most frequent histopathological findings in patients with RA and renal dysfunction. Systemic amyloidosis is the most frequent cause of nephrotic proteinuria and kidney failure in patients with RA. 4 The prevalence of clinical findings such as hematuria has been reported in between5 % and 35 %; and proteinuria, in between 3 % and 7 %.
Renal dysfunction is occasionally intractable. Some patients develop chronic kidney failure and eventually require renal replacement therapy. This type of insufficiency is one of the main prognostic factors in patients with RA. 5
The current prevalence of chronic kidney failure is difficult to establish, given the numerous factors that interfere with renal function of patients with RA. Kidney failure as a cause of death has been reported in between 3% and 20% of cases.6,7 However, these studies do not reflect the true extent of kidney disease in patients with RA and there are no controlled clinical studies to determine the incidence of renal disease in patients with RA.
The objective of this study was to describe the histopathological findings in renal biopsies of patients with RA and correlate them with the clinical and laboratory manifestations (including the form of presentation, urinary sediment, and treatment with gold salts and non-steroidal anti-inflammatory drugs - NSAIDs). Follow-up was carried out during 12 months in order to evaluate which glomerulopathies showed worse evolution, based on the glomerular filtration rate calculated through the formula of MDRD of 4 variables.
Materials and methods
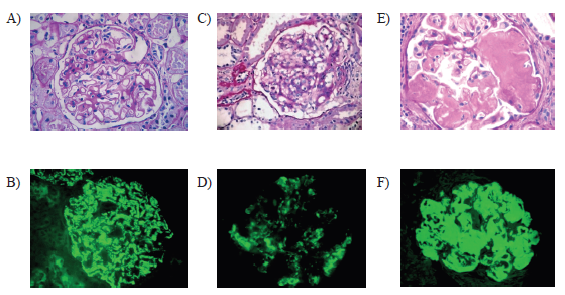
The renal biopsies of the patients with RA of the division of nephrology of the Hospital de Clínicas José de San Martín (HCJSM), of the city of Buenos Aires, were analyzed. These biopsies were done between January 1989 and January 2010. The diagnosis of RA was established according to the American College of Rheumatology 1987 criteria. Different glomerular diseases were found in a total of 41 patients.Mesangial glomerulopathy (MGP), glomerulonephritis with extracapillary proliferation(GNEC), membranous nephropathy (MN), secondary amyloidosis (AA), focal and segmental glomerulosclerosis (FSGS), and minimal change disease (MCD) were included.During the review, we analyzed the years of evolution of the RA, the treatments received with gold salts and non-steroidal anti-inflammatory drugs (NSAIDs), the clinical presentation, histopathological findings, interstitial fibrosis and tubular atrophy. The latter was considered moderate when the commitment was more than 25%; and severe, when it was greater than 50%.
The 24 hour-proteinuria, plasma creatinine and glomerular filtration were calculated through the formula of MDRD of 4 variables. The urinary sediment was analyzed by nephrologists trained in this practice.
On the other hand, dysmorphic hematuria was defined as the presence of 3 or more red blood cells per high-power field (40x) and dysmorphism of more than 40%.8,9 Follow-up was carried out for 12 months, with the objective of observing which group had worse evolution, according to the measurements with the MDRD formula. (Figure 1)
Inclusion criteria:
Diagnosis of RA, according to the criteria of the American College of Rheumatology
Renal biopsy with more than 10 glomeruli
Patients older than 18 years
Statistical analysis
The qualitative variables were studied through relative and absolute frequencies; and the quantitative variables, with arithmetic means and standard deviations. The different variables of laboratory and clinical presentation were analyzed. Likewise, we analyzed the evolution at 6 months and 12 months of 24-hour proteinuria, plasma creatinine and MDRD.
Patients with a diagnosis of RA according to criteria of the American College of Rheumatology, and who had a biopsy with glomerular disease in the period between January 1989 and January 2010, were included. A complete follow-up of the medical records was carried out until 12 months. The urine sediment was analyzed by nephrologists from the HCJSM service. Patients who showed a coexistence of other rheumatological diseases and renal biopsies without immunofluorescence were excluded.
Results
The population under study was of female gender in 68% (n = 28) and of male gender in 32% (n = 13). The most frequent histological finding was amyloidosis (34.1 %, n=14), followed by mesangial glomerulopathy (21.9 %, n=9), membranous nephropathy (19.5 °%, n=8), glomerulonephritis with extracapillary proliferation (12.1 %, n=5), focal and segmental glomerulosclerosis (7.3 %, n=3), and minimal change disease (4.87 %, n=2). The glomerulopathies that showed greater proteinuria at the beginning were membranous nephropathy, amyloidosis and minimal change disease.Both membranous nephropathy and minimal change disease had partial remission at one year, unlike amyloidosis, which showed progression of proteinuria at 12 months of follow-up.
Regarding the forms of clinical presentation and urinary findings, 48.7% (n = 20) occurred as nephrotic syndrome. MCD and AA were the glomerulopathies with greater frequency. On the contrary, the presentation of microhematuria was more frequent in MGP, in 100% of cases (n = 9); and in GNEC, in 80% (n = 4).
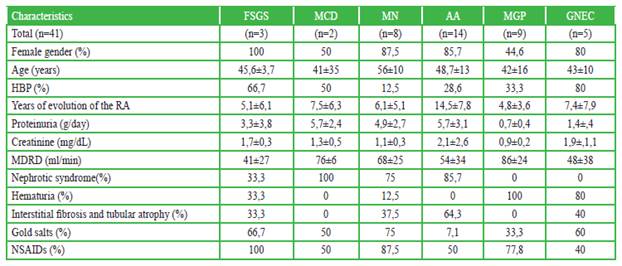
Regarding the time of evolution, the number of years was higher for patients with AA, with approximately 15 years (14.5 ± 7.8). It occurred similarly in MCD and GNEC, with approximately 7.5 years (7.5 ± 6.3 and 7.4 ± 7.9, respectively). (Table 1)
Table 1 Demographic characteristics, laboratory and histopathological findings in patients with rheumatoid arthritis
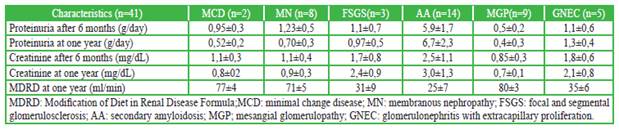
Amyloidosis, glomerulonephritis with extracapillary proliferation, and focal and segmental glomerulosclerosis) were the glomerulopathies that presented greater progression of renal failure at one year of follow-up (based on the estimation by MDRD); and which had greater tubulointerstitial involvement in renal biopsy (Table 2).
Table 2 Evolution of glomerulopathies in rheumatoid arthritis after one year
There are few studies describing the deterioration of renal function in patients with RA. A cross-sectional cohort study of 604 Finnish patients with RA documented nephropathy (defined as microhematuria, proteinuria or renal failure) in 17% of cases. Of the 604 patients, 54 had isolated hematuria, 27 had isolated proteinuria and 7 had microhematuria and proteinuria. Chronic renal failure defined by serum creatinine levels was found in a total of 29 patients, and 15 of them did not have hematuria or proteinuria. 1
In our study, proteinuria measured in 24 hours, plasma creatinine and glomerular filtration estimated by MDRD4 were analyzed at the time of the biopsy and at 6 and 12 months. It was evidenced that patients who had amyloidosis presented a higher degree of increase in proteinuria and renal failure at 6 and 12 months. Likewise, it was observed that there was a decrease in the glomerular filtration rate, evolutionarily, at 6 and 12 months for FSGS. However, it was not possible to made statistical comparisons due to the small size of the sample.
The limitations of our study are clear, since there was a small sample size, a retrospective approach and a follow-up of only 12 months. However, data were obtained from a local population.
Conclusions
The renal diseases reported in the literature and more commonly observed in patients with rheumatoid arthritis who underwent renal biopsy are mesangial glomerulopathy, amyloidosis and membranous nephropathy, whose correlation is maintained in our study.
The urinary sediment is a useful tool, because the presence of glomerular microhematuria is more prevalent in some entities than in others (greater in mesangial glomerulopathy and glomerulonephritis with extracapillary proliferation, in our cohort). The glomerulopathies that presented greater progression of renal failure at one year, based on the estimation by MDRD 4, showed a greater tubulointerstitial compromise in the renal biopsy. These were amyloidosis, focal segmental glomerulosclerosis and glomerulonephritis with extracapillary proliferation. Mesangial glomerulopathy, minimal change disease and membranous nephropathy were those with better evolution.
Ethical responsibilities
Protection of people and animals
The authors declare that no experiments were performed on human beings or animals for this research.
Data confidentiality
The authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consent
The authors state that patient data do not appear in this article.
Contribution of the authors
Preliminary project, project, data collection and analysis: Yaroslad De La Cruz, Cintia Marín, Martín Zapata, Luis José Daza, John Galindo.
Tematic and methodological tutor: Fernando Segovia.
Methodological and statistics tutor: Dr. José Lucas Daza.
Pathology tutor: Graciela De Rosa.
References
1. Karstila K, Korpela M, Sihvonen S, Mustonen J. Prognosis of Clinical Renal Disease and Incidence of New Renal Findings in Patients with Rheumatoid Arthritis: Follow-Up of a Population-Based Study. Clin Rheumato. 2007; 26(12):2089-2095. http://doi.org/10.1007/s10067-007-0625-y.
[ Links ]
2. Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL. Development of Reduced Kidney Function in Rheumatoid Arthritis. Am J Kidney Dis. 2014; 63(2):206-213. http://doi.org/10.1053/j.ajkd.2013.08.010.
[ Links ]
3. Yoshinaga Y, Nishiya K, Yamamura M, Hatano M, Ogura T, Takaoka M, et al. Study of Urinary findings and Renal Functions in Patients with Rheumatoid Arthritis [en Japonés]. Kidney Dialysis. 1989;26:477-482.
[ Links ]
4. Hill AJ, Thomson RJ, Hunter JA, Traynor JP. The Prevalence of Chronic Kidney Disease in Rheumatology Outpatients. Scott Med J. 2009; 54(2):9-12. DOI: http://doi.org/10.1258/rsmsmj.54.2.9.
[ Links ]
5. Symmons DP, Jones MA, Scott DL, Prior P. Long Term Mortality Outcome in Patients with Rheumatoid Arthritis: Early Presenters Continue to Do Well. J Rheumatol. 1998; 25(6):1072-1077.
[ Links ]
6. Laasko M, Mutrru O, Isomaki H, Koota K. Mortality from Amyloidosis and Renal Disease in Patients with Rheumatoid Arthritis. Ann Rheum Dis. 1986; 45(8):663-667.
[ Links ]
7. Adu D, Tse WY. Rheumatoid Arthritis, Mixed Connective Tissue Disease, and Polymyositis. in: Adu D, Emery P, Madaio MP, editores. Rheumatology and the Kidney. New York: Oxford University Press; 2001. p. 293-302.
[ Links ]
8. Fogazzi G. The Urinary Sediment. An Integraded View. 3a ed. Milan: Elsevier; 2009.
[ Links ]
9. Fogazzi GB, Grignani S: Urine microscopic analysis: An art abandoned by nephrologists? Nephrol Dial Transplant. 1998; 13:2485-2487.
[ Links ]
10. Hall CL, Fothergill NJ, Blackwell MM, Harrison PR, MacKenzie JC, MacIver AG. The Natural Course of Gold Nephropathy: Long Term Study of 21 Patients. Br Med J (Clin Res Ed). 1987; 295(6601):745-748.
[ Links ]
11. Hall CL, Jawad S, Harrison PR, MacKenzie JC, Bacon PA, Klouda PT, MacIver AG. Natural Course of Penicillamine Nephropathy: A Long Term Study of 33 Patients. Br Med J (Clin Res Ed) . 1988; 296(6629):1083-1086.
[ Links ]
12. Bourke BE, Woodrow DF, Scott JT. Proteinuria in Rheumatoid Arthritis-Drug-Induced or Amyloid? Ann Rheum Dis . 1981; 40(3):240-244.
[ Links ]
13. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal Biopsy Findings and Clinicopathologic Correlations in Rheumatoid Arthritis. Arthritis Rheum. 1995; 38(2): 242-247.
[ Links ]












 texto en
texto en 



