Introduction
Cancer is one of the major diseases responsible for high mortality worldwide. Oral squamous cell carcinoma (OSCC) is the most common cancer in the oral cavity and ranked 6th and 10th commonest in males and females worldwide respectively 1,2. In Iraq, studies showed a rapid increase in the incidence of OSCC 3. On clinical inspection by a dentist, it is comparatively easy to identify a tumor by visual examination or through some OSCC tumor markers, but due to ignorance by the patients at the early stage led to the progression of this tumor, and subsequently, OSCC would be diagnosed at the advanced stages which then lead to high mortality 4. Surgery and chemotherapy fail to cure or prevent the recurrence and metastasis of tumors and are often associated with side effects; thus, many researchers are focused on natural products to discover many new drugs for oral cancer treatment from some potential candidates 5,6,7. Natural products are well flourished for therapeutic actions preventing and treating different ailments 8-10. Many new anticancer therapies from natural products were reported in basics researches 11. In general preventive practice, cytotoxic and hemolytic properties of natural sources were screened to predict the possible toxic effects on mammalian cells before developing them into a therapeutic agent for future work 8,9.
Moringa peregrina Forssk is a well-known, traditionally used plant grown in Africa and Asia 10. M. peregrina seeds contain highly pharmacologically active components such as oleic acid, linoleic acid, isothiocyanate, tocopherols, flavonoids, and phenolic compounds. These compounds are reported to have different biological activities like antidiabetic 11, anti-Herpes simplex virus 12, anti-inflammatory 13, antibacterial 14, and anticancer against various cell lines (CACO-2, MCF-7, HeLa, HepG2, and L929) 16.
Even though several pharmacological studies on M. peregrina seeds have been reported in the literature, the anti-proliferation effect of M. peregrina seeds against oral carcinoma cells and the evaluation of the hemolytic capacity towards erythrocytes have not been scientifically reported yet. Thus, this study was conducted to test cytotoxic activities on the CAL 27 cell line and the effect of MPSE on human erythrocytes.
Materials and methods
Preparation of plant
In 2013, M. peregrina seeds were collected and submitted at the Department of Botany, University of Khartoum, where Dr. Maha Kordofani, a resident botanist, authenticated these seeds. These M. peregrina seeds were air-dried for 5 days at 25-30oC, powdered, and macerated in 1:5 dried plant weights to ethanol volume ratio. The collected filtrate was dried under reduced pressure at 46 to 51°C using a rotary evaporator to obtain crude M. peregrina seed extract (MPSE) 9.
Cytotoxicity Analysis Using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) colorimetric assay
The MPSE was tested on the CAL 27 Cell lines to check the cytotoxic activity of this extract. CAL 27 Cell line was grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% penicillin/streptomycin, 10% FBS, and in a humidified 5% CO2 chamber at 37°C; the cells were seeded at 5 × 103 cells/well into 96-well plates. The vehicle used to prepare the initial stock of MPSE was 0.1% dimethylsulfoxide (DMSO). After 24 h, these cells were treated with MPSE at different concentrations (12.5-100 μg/mL) and incubated for 48 h. After incubation, cell viability was measured by adding 20 µL of MTT dye (5 mg/mL) to all wells and further incubated for 4 h. The percentage of cytotoxicity of the extract was calculated by measuring the absorbance of each well at a wavelength of 540 nm using an ELISA plate reader (Tecan, California, USA) 15.
Hemolytic Activity
After getting approval from the Independent Ethics Committee of ICCBS, University of Karachi, with reference no ICCBS/IEC-047-HB-2019/Protocol/1.0, the hemolytic assay was carried out.
The hemolytic effect of MPSE was determined using the method described by Wiradharma et al., 2011 16. Human blood (3 mL) withdrawn from a volunteer was collected in an EDTA-anticoagulated venoject tube and centrifuged at 700 ×g, and the packed erythrocytes were separated from the plasma. The erythrocytes were washed thrice with 1X PBS and then diluted with PBS to obtain a 4% erythrocyte suspension. 100 µL of erythrocyte suspension were placed in triplicates in the wells of the 96-well cell culture plates. The erythrocytes in the respective wells were treated with 250, 500, and 1000 µg/mL of MPSE, and the plate was incubated at 37ºC for 1 h to allow for hemolysis. The plate was then centrifuged at 800 ×g for 15 min. From each well, 100 µL of supernatants were transferred to a new 96-well plate, and the absorbance was determined in a microplate reader (MultiSkanGo, Thermo Scientific) at 576 nm. Erythrocytes treated with Triton X-100 (0.5%) and PBS served as the positive and negative control, respectively. Erythrocytes in 2% DMSO were the solvent control.
Results
Effects of MPSE on CAL 27 Oral cancer cell line
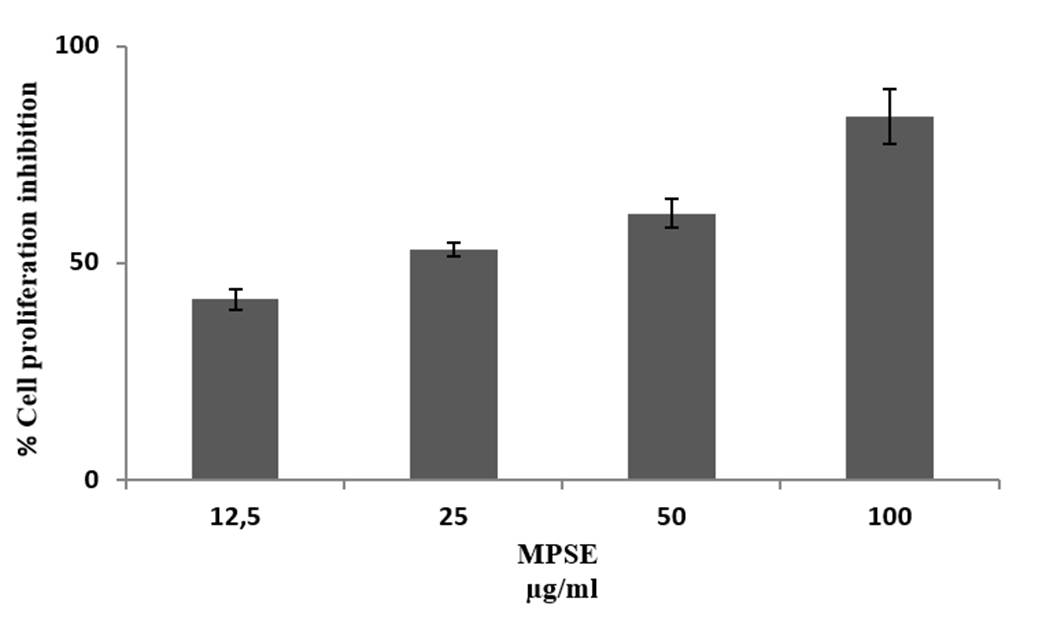
MPSE inhibited in vitro proliferation of CAL 27 cell lines. At 100 µg/mL, MPSE showed more than 80% inhibition (Figure 1). IC50 of MPSE was found to be 25 µg/mL, significantly higher than doxorubicin IC50 = 0.5 μg/mL 15. The extract was active below 100 µg/mL, demonstrating the presence of the potential compounds in the extract and showing anti-cancer activity.
The hemolytic effect of MPSE on human erythrocytes
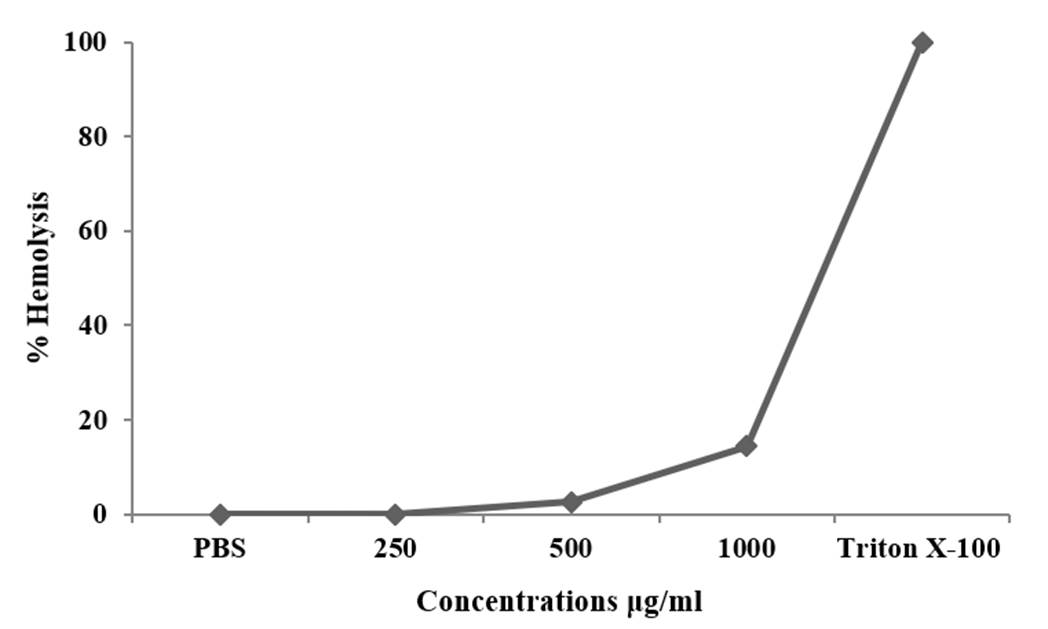
The hemolytic effect of MPSE augmented with increasing treatment concentrations. MPSE caused 14.3 % hemolysis at 1,000 μg/mL. This hemolysis was below 20%, demonstrating the non-hemolytic property of this extract. The positive (Triton X-100 (0.5%) control showed full hemolysis (100%), while the negative control (PBS-treated RBC cells) did not observe hemolysis (0%) (Figure 2).
Discussion
Since ancient times, people from distinct civilizations have used different plants from their surroundings to treat various disorders. Recently, the emergence of multidrug-resistant diseases, such as MDR pathogens, cancer, diabetes, and other inflammatory diseases, has motivated researchers to explore different natural sources, such as animals and plants, to find novel compounds to treat various conditions. To date, many antibacterial, anticancer compounds isolated from natural sources have been extensively used due to their effective therapeutic use 17-21. Recently, M. peregrina seed extracts have gained popularity due to their antioxidant 11, anti-inflammatory 13, antibacterial 14, anticancer 15, antispasmodic 14 activities.
M. peregrina seed have been reported for its cytotoxic effect on CACO-2, HeLa, AU565, MCF-7, L929, HepG2, PC-3 cell lines 15,19. Abou-Hashem et al. 2019 19 reported the chloroform fraction of the ethanolic extract of M. peregrina seed extract as the most active antitumor fraction. HPLC analysis of this chloroform fraction reported many polyphenols like vanillin, syringic acid, naringenin, ferulic acid, quercetin, coumaric acid, daidzein, and cinnamic acid. Syringic acid is reported to have an antimitogenic effect on human colorectal cancer cells 20. Coumaric acid 23 and vanillin 24 also inhibit colon cancer cells by inhibiting the cell cycle and inducing apoptosis. Naringenin and ferulic acid inhibit gastric cancer cells and osteosarcoma cells through apoptosis by downregulating the protein kinase (AKT) pathway 25,26. Daidzein induced apoptosis and cell cycle arrest in human ovarian cancer cells 27. Cinnamic acid inhibits human melanoma cells through apoptosis by disrupting the cytoskeleton 28. This fraction also contains flavonoids like quercetin which interacts with DNA and activates the mitochondrial pathway of apoptosis in leukemia cancer cells 29. All these phenolic and flavonoid compounds were reported to have an anticancer effect on different cancer cell lines; therefore, these compounds may act synergistically on different targets of the complex cellular pathway and induce cytotoxicity toward cancer cells. These compounds induce apoptosis by activating various apoptotic pathways like upregulating caspase3 with the release of cytochrome c, downregulating anti-apoptotic genes (Bcl-2, Bcl-xL), cell cycle arrest, premature aging, activating mitochondrial apoptosis pathway, by enhancing the immune system to destroy cancer cells, reducing proliferation, angiogenesis, differentiation and metastasis of cancers 30-32.
Although many anticancer compounds had potent anticancer activity against various cancer cell lines, they failed to advance in clinical trials because they were cytotoxic to normal host cells and blood cells. Some were highly hemolytic, limiting them to topical use. Therefore, it is necessary to verify in vitro the hemolytic effect of M. peregrina on human erythrocytes since most anticancer agents damage blood cells, causing anemia and myelosuppression 33. Any compounds, formulations, or extracts with less than 10% hemolysis are non-hemolytic, and those with more than 25% are at risk of hemolysis 34.
Therefore, for the suitability of any compounds, formulation, or extracts to treat diseases, hemolysis must be less than 25%. In the present study, MPSE showed a maximum of 14.3% hemolysis when treated at 1,000 μg/mL, which is below 25%. Thus, this extract showed non-hemolytic activity and can be used for oral consumption for treating various diseases.