Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.35 no.2 Bogotá July/Dec. 2009
Selectivity of insecticides for adult workers of Apis mellifera (Hymenoptera: Apidae)
Selectividad de insecticidas sobre obreras adultas de Apis mellifera (Hymenoptera: Apidae)
DANIELLE THOMAZONI1, MIGUEL F. SORIA2, CÁSSIO KODAMA2, VLADSON CARBONARI1, RONI P. FORTUNATO2, PAULO E. DEGRANDE1 and V. A. Jr., VALTER1
1 Universidade Federal da Grande Dourados, Programa de Pós-graduação em Entomologia e Conservação da Biodiversidade, Faculdade de Ciências Biológicas e Ambientais, Departamento de Ciências Biológicas/Unidade II. Rodovia Dourados-Itahum, Km 12, Caixa Postal: 241, Cep: 79804-970, Dourados/MS, Brazil.
2 Universidade Federal da Grande Dourados, Programa de Pós-graduação em Produção Vegetal, Faculdade de Ciências Agrárias, Departamento de Ciências Agrárias/Unidade II. Rodovia Dourados-Itahum, Km 12. Cidade Universitária. Aeroporto. Caixa Postal: 533, CEP: 79.804-970, Dourados, MS, Brasil. paulodegrande@ufgd.edu.br. Autor para correspondencia.
Received: 14-sep-2008 - Accepted: 11-jul-2009
Abstract: Cotton is a crop that is attractive to a wide variety of organisms, including pests, natural enemies of the pests and arthropod pollinators. Consequently, integrated pest management is necessary for this crop as chemical control is often required to avoid production losses. Among pollinators found on cotton, Apis mellifera is one of the most important, as it can increase cotton production 20 - 30%. On the other hand, selectivity is an important characteristic of insecticides, as it makes it possible to lessen impacts on pollinators and other non-target organisms. We examined the toxicity of various insecticides for honey bees foraging on cotton in a greenhouse, including Turbine® 500 WG (150 g ha-1) (flonicamid), Actara® 250 WG (200 g ha-1) (thiamethoxam), Cartap® 500 SP (1500 g ha-1) (cartap), Talstar® 100 CE (1000 mL ha-1) (bifenthrin) and Match® 50 CE (1000 mL ha-1) (lufenuron). Applications of Talstar®, Actara® and Cartap® were quite toxic. Talstar® was the most deadly, while Match CE® and Turbine® were only slightly toxic for adult honey bees. The Match CE® treatment mortality was similar to that of the control; thus, we classified it as innocuous for A. mellifera adults.
Key words: Pollinator. Non-target effects. Environmental impact.
Resumen: El algodón es un cultivo atractivo a una amplia variedad de organismos incluyendo plagas, enemigos naturales de las plagas y los polinizadores. Por lo tanto, el manejo integrado de plagas es necesario para este cultivo ya que el control químico se requiere a menudo para evitar pérdidas de producción. Entre los polinizadores encontrados en algodón, Apis mellifera es uno del más importantes pues puede aumentar la producción de 20 - 30% del algodón. Por otro lado, la selectividad es una característica importante de insecticidas, pues permite disminuir impactos en polinizadores y otros organismos no-blanco. Se examinó la toxicidad de varios insecticidas sobre abejas de Apis mellifera forrajeando en el algodón en condiciones de invernadero, incluyendo Turbine® 500 WG (150 g ha-1) (flonicamid), Actara® 250 WG (200 g ha-1) (thiametoxam), Cartap® 500 SP (1500 g ha-1) (cartap), Talstar® 100 CE (1000 ml ha-1) (bifenthrin) y Match® 50 CE (1000 ml ha-1) (lufenuron). Las aplicaciones de Talstar®, Actara® y Cartap® fueron absolutamente tóxicos. Talstar ® fue el más mortal, mientras que el Match® CE y Turbine® fueron solamente levemente tóxicos para los adultos de la abeja. El tratamiento Match® CE presentó mortalidad similar a la del control; entonces, lo clasificamos como inofensivo para los adultos de A. mellifera.
Palabras clave: Polinizadores. Efecto no blanco. Impacto ambiental.
Introduction
Although cotton (Gossypium hirsutum L.) is attacked by agricultural pests that damage roots, stems, leaves, flower buds, flowers, fruits and bolls, it is also host to various pollinators and natural enemies such as predators, parasitoids and pathogens, which are indispensable components of dynamic agroecosystems. Damage inflicted by agricultural pests can drastically reduce cotton productivity, directly affecting seed and fiber characteristics, and reducing their commercial value (Degrande and Gomes 1990). Consequently, pest control techniques, such as cultural, chemical and biological tactics that are part of Integrated Pest Manegement (IPM) of cotton, have proven to be extremely important for the success of this crop. Their use has increased significantly as it is considered a safe methodology that provides a satisfactory cost/benefit ratio (Degrande 2000; Gallo et al. 2002).
Among control methods, chemicals are the most widely employed due to their ease of application and effectiveness (Santos 1999). However, careless use of this form of control results in adverse effects on non-target organisms, including humans, domestic animals, pollinators, natural enemies and other wild organisms, in addition to contaminating soil and water. These side effects need to be eliminated or minimized in order to avoid problems such as pest reinfestations, secondary pest outbreaks, and evolution of pest resistance (Metcalf 1982; Nakano 1986).
Among the beneficial pollinator insects that visit cotton, the Africanized honey bee, Apis mellifera L., 1752 (Hymenoptera: Apidae), is prominent. In addition to its ecological importance as a pollinator, this insect has its own economic importance as a producer of honey, beeswax and royal jelly. Some studies have demonstrated an increase in cotton fruit and seed production on the order of 20 - 30% when honey bees pollinate this crop, which has also been found for other crops, such as sunflower and onion seed (Fermiano 1981; Lorenzon and Martinho 1992; Moreti et al. 1996; Gallo et al. 2002). Laboratory tests of the effect of insecticides pyrethroids are the most dangerous (100% mortality within two hours after spraying), while carbamates and organophosphates elicited 50% mortality under the same conditions (Rigotti 2005).
If insecticides are used in an appropriate manner, they can control target organisms without negatively affecting natural enemy and pollinator populations; otherwise, these predators and pollinators will be less able to provide their beneficial services (Melksham et al. 1988; Davis 1989). One way to achieve this objective would be to use selectively toxic insecticides (Carvalho et al. 2002; Degrande et al. 2002; Magalhães et al. 2002; Godoy et al. 2004). Considering the importance of A. mellifera as a pollinating agent in agroecosystems, we evaluated the effect of the insecticides Turbine® 500 WG (flonicamid), Actara® 250 WG (thiamethoxam), Cartap® 500 SP (cartap), Talstar® 100 CE (bifenthrin) and Match 50 CE® (lufenuron), which are used on cotton, on the mortality of honey bee workers.
Material and Methods
The experiment was conducted in a greenhouse, with potted cotton plants at the Faculdade de Ciências Agrárias (FCA) of the Universidade Federal da Grande Dourados (UFGD), in Dourados (22°14’S, 54°44‘W, altitude 452 m), Mato Grosso do Sul state, Brazil, from November 2006 to January 2007. Each pot was planted with six seeds of cotton cultivar Fibermax 933, later reduced to four plants per pot. The soil used for the plants’ cultivation was an Oxisol collected from the subsoil (> 40 cm deep). During flowering, approximately 50 - 55 days after germination, the plants were sprayed with the insecticide treatments at a rate of 400 liters of spray liquid per hectare. The sprayer was washed with water, water + detergent and 92.8% ethanol between treatments, to remove residues of the different insecticides.
The experimental design consisted of randomized blocks, with six treatments and four replicates per treatment. Each plot consisted of a pot with four plants and 30 A. mellifera adult workers, which were confined in gauze cages 98.5×41 cm. The treatments were: 1) Turbine® 500 WG (150 g/ha) (flonicamid), 2) Actara® 250 WG (200 g/ha) (thiamethoxam), 3) Cartap® 500 SP (1500 g/ha-1) (cartap), 4) Talstar® 100 CE (1000 mL/ha-1) (bifenthrin), 5) Match 50 CE® (1000 mL/ha) (lufenuron) and 6) a control sprayed with water; in every case a manual sprayer at a constant pressure was used. Spraying began at 9:00 h, at 29ºC, 68% relative humidity and wind speed below 2.5 m s-1, at a rate of 112 L/ha-1, these conditions are those usually adopted in the field for insecticide sprays. The spray was allowed to dry on the plants for three hours before adding the 30 bees per pot. Four replicates were made for each insecticide treatment. Each group of bees was provided with PET bottle caps containing water and with a mixture of honey plus sucrose composed of 50% honey or water and 50% refined sugar (Wiese 1995).
The A. mellifera workers were estimated to be about 5-6 days old, based on their coloration and hair density on the thorax, plus their in-colony activities (Free 1980). The bees were collected from two colonies located in apiaries near the university (FCA/UFGD), using a manual aspirator, and were maintained in a B.O.D incubator (28±1°C and 12 hours photophase) for 24 hours; they were fed with the sucrose and honey mixture plus water until they were placed in the gauze cages in the greenhouse. Evaluations were made every 30 minutes for six hours, counting the number of bees that had died. For each evaluation the data were tabulated, expressed as percentage mortality and transformed with square root of (x+0.5) for the statistical analysis. The means were compared by the Tukey’s test (P < 0.05).
Results and Discussion
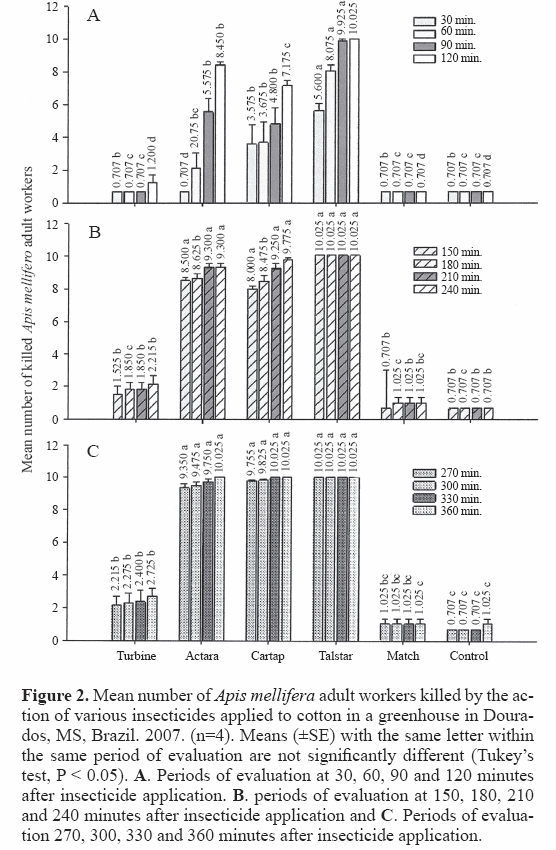
Among the six insecticide treatments, Talstar® 100 CE (1000 mL ha-1) was the least selective, causing 100% mortality of A. mellifera workers in the shortest time (Figs. 1 and 2). This product is similar to the pyrethroid bifenthrin, which acts on the modulation of Na+ channels in the central and peripheral nervous systems of insects. It has a potent “knock-down” effect, and after contact with this insecticide, the workers displayed uncoordinated movements and trembling, manifesting the typical effects of pyrethroid insecticides, also observed by Carvalho (2006). Rigotti (2005) also found that pyrethroidbased insecticides used commercially on soybeans caused 100% mortality of A. mellifera adults, within two hours of spraying in the lab.
Treatments with the insecticides Actara® 250 WG (200 g ha-1) (thiamethoxam) and Cartap® 500 SP (1500 g ha-1) (cartap), which affect the insect nervous system, were also not selective, although they killed the bees at a slower rate than did bifenthrin. Total mortality (100%) of bee workers occurred after 360 minutes with Cartap® and after 330 minutes with Actara® (Fig. 1). Carvalho et al. (2004 a,b) and Carvalho (2006) reported similar effects on A. mellifera workers, independent of the mode of application whether direct spraying or incorporation in the diet; thiamethoxam was highly toxic to bees, requiring a mean of 22.5 hours to kill 100% of the workers. High toxicity of thiamethoxam was also found by Iwasa et al. (2004), who reported an LD50 of 0.03 μg/bee. According to Karnatak and Neetu (2005), thiamethoxam is one of the most toxic insecticides for A. mellifera.
Similar results were reported by Thompson (2003) and Rhodes and Scott (2006), who classified thiamethoxam as highly toxic. They also indicated that this insecticide can elicit physiological and behavioral effects in the bees, when applied at sublethal doses. These include alterations in the embryonic, larval and pupal phases, as well as changes in flight activity, negatively affecting food collection and consequently colony development.
Treatments with the insecticides Turbine® (150 g ha-1) (flonicamid) and Match 50 CE® (1000 mL ha-1) (lufenuron) were less toxic for A. mellifera workers. Match 50 CE® did not significantly affect the bees and could be considered innocuous for them (Figs. 1 and 2). Similar results were found with the predator wasp Polistes canadensis canadensis (L., 1758) (Santana-Reis et al. 2002) in laboratory tests with lufenuron. This is a chitin-synthesis regulator, acting on ecdysis during the insect larval stages.
Based on our greenhouse evaluations, the insecticide Turbine® was considered selective or relatively non-toxic for bees, as was Match CE®, eliciting only 9.16% mortality (Figs. 1 and 2). Carvalho (2006) also tested lufenuron directly on bees in the lab and found low toxicity, similar to that found for the control, reaching 21% after 72 hours of confinement, and similar to what was reported by Atkins et al. (1981), Tew (1996) and Hunt (2000).
Conclusions
Spraying with Talstar®, Actara® and Cartap® was lethal for A. mellifera; Talstar® was the most acutely toxic. Match CE® and Turbine® were less toxic; mortality due to Match CE® was similar to that obtained with the control spray. We classified Match CE® as innocuous for adult honey bees under these conditions. This research contributes information that can help conserve A. mellifera populations in the vicinity of cotton crops, contributing to the productivity of the cotton crop and to the quality of bee products in neighboring apiaries.
Literature cited
ATKINS, E. L., KELLUM, D.; ATKINS, K. W. 1981. Reducing pesticides hazardous to honeybees. Mortality prediction techniques and integrated management strategies. Berkeley: University of California. 20 p. [ Links ]
CARVALHO, G. A.; CARVALHO, C. F.,; SOUZA, B.; ULHÔA, J. L. R. 2002. Seletividade de inseticidas a Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotropical Entomology 31 (4): 615-621. [ Links ]
CARVALHO, S. M.; CARVALHO, G. A.; CARVALHO, C. F.; BRIGHENTI, D. M. 2004a. Toxicidade de produtos fitossanitários utilizados em citros sobre operárias de Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae), pp. 547. In: Congresso Brasileiro de Entomologia 20, 2004, Gramado. Proceedings. Gramado. [ Links ]
CARVALHO, S. M.; CARVALHO, G. A.; CARVALHO, C. F.; MOSCARDINI, V. F. 2004b. Efeito de produtos fitossanitários recomendados para a cultura de citros sobre operárias de Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae) fornecido por meio de pasta Cândi contaminada, pp. 547. In: Congresso Brasileiro de Entomologia 20, 2004, Gramado. Proceedings. Gramado. [ Links ]
CARVALHO, S. M. 2006. Toxicidade de produtos fitossanitários utilizados na cultura de citros a operárias de Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae). M.Sc. thesis - Universidade Federal de Lavras. 72 p. [ Links ]
DAVIS, A. R. 1989. The study of insecticide poisoning of honeybee brood. Bee World 4: 163-174. [ Links ]
DEGRANDE, P. E. 2000. Manejo de pragas: realidade e desafios, pp. 229-244. In: Congresso Internacional do Agronegócio do Algodão/ V Seminário Estadual da Cultura do Algodão: Negócios e Tecnologias para melhorar a vida. Proceedings of the International Congress of Cotton Agribusiness. Cuiabá-MT-Brasil.. [ Links ]
DEGRANDE, P. E.; GOMES, D. R. S. 1990. Seletividade de produtos químicos no controle de pragas. Agrotécnica: 8-13. [ Links ]
DEGRANDE, P. E.; REIS, P. R.; CARVALHO, G. A.; BELARMINO, L. C. 2002. Metodologia para avaliar o impacto de pesticidas sobre inimigos naturais, pp. 71-93. In: Parra, J. R. P.; Botelho, P. S. M.; Corrêa-Ferreira, B. S.; Bento, J. M. S. Controle Biológico no Brasil. São Paulo, Manole. [ Links ]
FERMIANO, L. H. M. N. 1981. Estudo do comportamento de coleta de alimento em duas subespécies de Apis mellifera (Hymenoptera, Apidae) e nos descendentes F1 resultantes de seu cruzamento. M.Sc. thesis. FMRP-USP, 81 p. [ Links ]
FREE, J. B. 1980. Organização social das abelhas (Apis). Edusp. 79 p. [ Links ]
GALLO, D.; NAKANO, O.; SILVEIRA, S.; CARVALHO, R.; BAPTISTA, G.; BERTI, E.; PARRA, J. R.; ZUCCHI, R. A.; ALVES, S. B.; VENDRAMIM, J. D.; MARCHINI, L. C.; LOPES, J. S.; OMOTO, C. 2002. Entomologia Agrícola. Biblioteca de Ciências Agrárias Luiz de Queiroz. São Paulo: FEALQ. 920 p. [ Links ]
GODOY, M. S.; CARVALHO, G. A.; MORAES, J. C.; JÚNIOR, M. G.; MORAIS, A. A; COSME, L. V. 2004. Seletividade de inseticidas utilizados na cultura dos citros para ovos e larvas de Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotropical Entomology 33: 639-646. [ Links ]
HUNT, G. J. 2000. Using honey bees in pollination. Beekeeping: E- 216-W. Purdue University Cooperative Extension Service. 5 p. [ Links ]
IWASA, T.; MOTOYAMA, N.; AMBROSE, J. T.; ROE, R. M. 2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Protection 23 (5): 371-378. [ Links ]
KARNATAK, A. K.; NEETU, S. 2005. Relative toxicity of some insecticides to the workers of Apis mellifera L. Shashpa 12 (1): 23-25. [ Links ]
LORENZON, M. C. A.; MARTINHO, M. R. 1992. Polinizadores da floração da cebola em Igarapé, estado de Minas Gerais. Anais da Sociedade Entomológica do Brasil 3: 383-389. [ Links ]
MAGALHÃES, L. C.; GUEDES, R. N. C.; OLIVEIRA, E. E., TUELHER, E. S. 2002. Desenvolvimento e reprodução do predador Podisus distinctus (Stål) (Heteroptera: Pentatomidae) frente a doses subletais de permetrina. Neotropical Entomology 31: 445-448. [ Links ]
MELKSHAM, K., JACOBSEN, J. N., RHODES, J. 1988. Compounds which affect the behaviour of the honeybee, Apis mellifera L.: a review. Bee World 3: 105-124. [ Links ]
METCALF, R. L. 1982. Insecticides in pest management, pp. 217-277. In: Metcalf, R. L.; Luckman, W. H. (Ed.). Introduction to insect pest management. 2nd ed. New York: John Wiley.. [ Links ]
MORETI, A. C., SILVA, C. C.; SILVA, R. M. B.; SILVA, E. C. A. 1996. Aumento na produção de sementes de girassol (Helianthus annus) pela ação de insetos polinizadores. Scientia Agricola 2: 13-16. [ Links ]
NAKANO, O. 1986. Avanços na prática do controle de pragas. Informe Agropecuário 12: 55-59. [ Links ]
RHODES, J.; SCOTT, M. 2006. Pesticides: a guide to their effects on honey bees. NSW Department of Primary Industries: Primefacts 149. 4 p. [ Links ]
RIGOTTI, M. 2005. Efeito da pulverização de inseticidas utilizados na cultura de soja sobre adultos de Apis mellifera Linnaeus (Hymenoptera, Apidae) em condições de laboratório. M.Sc. thesis - Universidade Federal da Grande Dourados, 34 p. [ Links ]
SANTANA-REIS, V. P. G.; MARQUES, O. M., COSTA, J. C. 2002. Seletividade de inseticidas ao predador Polistes canadensis canadensis (L., 1758) Hymenoptera: Vespidae. Acta Biológica Leopoldensia 2: 141-146. [ Links ]
SANTOS, W. J. 1999. Monitoramento e controle das pragas do algodoeiro, pp. 133-179. In: Cia, E.; Freire, E. C.; Santos, W. J. dos. Cultura do algodoeiro. POTAFOS: Associação Brasileira para pesquisa da potassa e do fosfato. Piracicaba-SP. [ Links ]
TEW, J. E. 1996. Protecting honey bees from pesticides. Ohio: The Ohio State University (Ohio State University Extension. Circular HYG-2161-97). 10 p. [ Links ]
THOMPSON, H. M. 2003. Behavioural effects of pesticides in bees - their potential for use in risk assessment. Ecotoxicology 12 (1/4): 317-330. [ Links ]
WIESE, H. 1995. Novo Manual De Apicultura. Livraria Editora Agropecuária. 292 p. [ Links ]