Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.23 no.4 Medellín Oct./Dec. 2010
A respiration-metabolism chamber system for measuring gas emission and nutrient digestibility in small ruminant animals¤
Sistema de cámaras respiro-metabólicas para medición de gases y digestibilidad de nutrientes en pequeños rumiantes
Um sistema de câmara de respiração e metabolismo para medição de emissões de gás e digestibilidade de nutrientes em pequenos ruminantes
Dong Hua Li1,Animal Science, MS; Beob Gyun Kim1,Animal Science, PhD; Sang Rak Lee1*, Animal Science, PhD.
1Department of Animal Science and Environment, Konkuk University, Seoul 143-701, Republic of Korea.
(Received: 24 september, 2010; accepted: 3 October, 2010)
Summary
A novel chamber system was developed to measure gas emission from small ruminants and to measure nutrient digestibility simultaneously. Animal behavior can also be easily observed in this system through transparent panels. The system is composed of respiration-metabolism chambers, gas sampling and analysis units, and a data acquisition unit. A recovery test was performed using standard methane (CH4) gas. Values for recovery rate of CH4 gas ranged from 96.7 to 99.6% in 8 replications, and the mean value was 98.1% (coefficient of variation = 1.3%). A preliminary animal experiment was also conducted using 4 Korean native black goats with a mean body weight of 23.5 kg. The goats consumed a 50:50 mixture of forage and concentrate, and daily feed allowance was 2% body weight. Dry matter digestibility was 70.9%, and the mean CH4 gas production was 11.6 g/day that was calculated to be 24.7 g/kg dry matter intake. Using this system, researchers can accurately and efficiently conduct various experiments measuring methane emission, nutrient digestibility, or both in small ruminant animals including goats, sheep, and deer.
Key words: digestibility, methane, respiration-metabolism chamber system, small ruminant.
Resumen
Se ha desarrollado un sistema de cámaras para medir simultáneamente la emisión de gases de pequeños rumiantes y la digestibilidad de nutrientes dietarios. Gracias a que las cámaras cuentan con paneles transparentes, también se puede observar fácilmente el comportamiento animal. El sistema completo se compone de varias cámaras para respirometría y metabolismo, unidades de muestreo y análisis de gas, así como de una unidad de adquisición de datos. Tras una prueba de recuperación con metano (CH4), se observó que la tasa de recuperación de CH4 fluctuó entre 96.7 y 99.6% en 8 repeticiones, y el valor medio fue del 98,1% (coeficiente de variación = 1,3%). Un experimento preliminar con animales se llevó también a cabo con 4 cabras Coreanas negras nativas de 23.5 kg de peso. Las cabras consumieron una mezcla 50:50 de forraje y concentrado, con un suministro diario de alimento equivalente al 2% del peso corporal. La digestibilidad de la materia seca fue del 70.9%, y la producción media de CH4 fue del 11.6 g / día, correspondiente a 24.7 g / kg de consumo de materia seca. Mediante este sistema se pueden realizar con precisión y eficiencia diversos experimentos de medición de emisiones de metano, digestibilidad de nutrientes, o ambos, en pequeños rumiantes tales como cabras, ovejas y ciervos.
Palabras clave: digestibilidad, metano, sistema de cámaras de respiración y metabolismo, pequeños rumiantes.
Resumo
Um curioso sistema de câmara foi desenvolvido para a medição simultânea de emissões de gás para pequenos ruminantes e a digestibilidade de nutrientes. O comportamento dos animais pode ser facilmente observado no sistema a traves de panéis transparentes. O sistema está composto de câmaras de respiração e metabolismos, unidades de amostragem análises de gás e uma unidade de aquisição de datas. Um teste de recuperação foi desenvolvido usando um gás metano estandardizado (CH4). Os valores de recuperação de CH4 variou entre 96.7 a 99.6% em 8 replicações, e a media foi de 98.1% (coeficiente de variação = 1.3%). Um experimento preliminar foi realizado utilizando 4 cabras pretas nativas coreanas com uma media de peso corporal de 23.5 kg. As cabras consumiram uma mistura de forragem e concentrado 50:50, e um suplemento do 2% do peso corporal. A digestibilidade da matéria seca foi de 70.9%, e a media da produção de gás CH4 foi de 11.6 g/dia a qual foi calculada por 24.7 g/kg do consumo de matéria seca. Usando este sistema, pesquisas com exactidão e eficiência podem ser realizadas para a medição de emissões de metano, digestibilidade de nutrientes, ou ambas em pequenos ruminantes incluindo cabras, ovelhas e veados.
Palavras chave: digestibilidade, metano, sistema de câmara de respiraçao e metabolismo, pequenos ruminantes.
¤ To cite this paper: Dong Hua Lī, Beob Gyun Kim, Sang-Rak Lee. A respiration-metabolism chamber system for measuring gas emission and nutrient digestibility in small ruminant animals. Rev Colomb Cienc Pecu 2010; 23: 444-450.
* Corresponding author: Sang-Rak Lee.Department of Animal Science and Environment, Konkuk University, Seoul 143-701, Republic of Korea. Tel +822 4503696. E-mail: leesr@konkuk.ac.kr
Introduction
Global heating is of great concern, and greenhouse gases are potentially major contributors to the climate changes. Methane (CH4) is the second most important greenhouse gas next to carbon dioxide based on the quantity (Martin et al., 2008). The production of CH4 from global ruminant livestock production is estimated to be approximately 80 million tonnes per year (Beauchemin et al., 2008) and is responsible for at least 15% of the total CH4 from human activities including agriculture and industry (Moss et al., 1995).
Two major methods have been employed to measure CH4 emission from animals: 1) the respiration chamber method (McGinn et al., 2004; Beauchemin and McGinn, 2005), and 2) the sulfurhexafluoride tracer method (Swainson et al., 2007; Martin et al., 2008). The first method is generally used in the buildings, and the second is for grazing animals. When a large number of animals are employed for an experiment, the second method is more appropriate, but the chamber method is more frequently used due to the relatively low data variability (Klein and Wright, 2006; Suzuki et al., 2008).
While respiratory systems are relatively easily available for ruminants, systems for both quantifying gas emission and determining nutrient digestibility in small ruminant animals have been rarely documented (Iwasaki et al., 1982). The systems designed for large ruminant animals take large space and high cost, and they may be less efficient and potentially less accurate when used for small ruminant animals. Therefore, a novel chamber system was developed to measure CH4 gas emission from small ruminant animals and to determine nutrient digestibility simultaneously. A recovery test was performed using standard CH4 gas to verify full detection of gas, and a preliminary animal experiment was also conducted.
Materials and methods
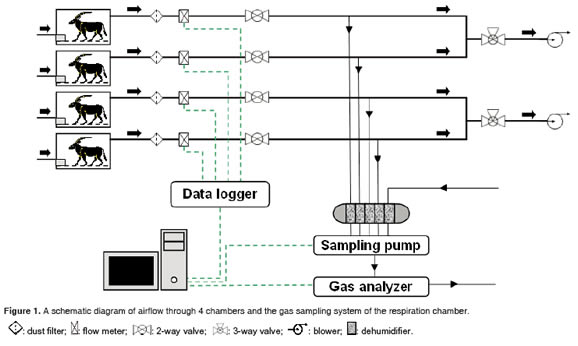
The respiration-metabolism chamber system was installed in an environmentally controlled room (ambient temperature, 23 °C; relative humidity, 55%) at Konkuk University. The protocols for animal experiments were reviewed and approved by Institutional Animal Care and Use Committee at Konkuk University. The system is composed of 3 major parts: 1) respiration-metabolism chambers, 2) gas sampling and analysis units, and 3) a data acquisition unit (Figures 1 and 2).
Respiration-metabolism chamber
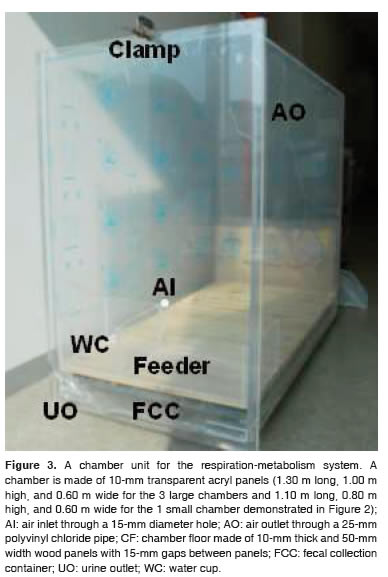
Each chamber was made of 10-mm transparent acryl panels (for the large 3 chambers in Figure 2, length, 1.30 m; height, 1.00 m; width, 0.60 m; Figure 3). The transparent materials were used to facilitate visual observation of animal behavior. All panels except the front panel were sealed using silicone sealant. The front panel was fastened to the other panels using clamps. A hole of 15-mm diameter on the front panel enabled air inlets into the chamber. On the back panel, another hole was made to connect a 25-mm polyvinyl chloride (PVC) pipe for gas outlets. A screen floor was made of 10-mm thick wood panels. The width of each panel was 50 mm, and the wood panels were spaced by 15 mm as an animal can comfortably stand on and feces and urine canefficiently pass through.Awatercupand afeeder were located on the top of the wood floor. Adrawertype container was placed below the wood floor to facilitate fecal collection. On the front bottom of the chamber,a15-mm hole for urine outlet was located.
Gas sampling and analysis unit
A ring blower (Model: GR40-610, Dongnam Engineering Co., Ansan, Korea) was equipped to draw air from 2 chambers through the PVC pipe at a rate of approximately 70 L/min (Figure 1). Based on our previous test, at this air flow rate, the carbon dioxide concentration in the chamber with a 25-kg goat did not exceed 0.5%, a suggested maximum concentration (Klein and Wright, 2006). The outlet air from a chamber was filtered through an air filter (Model:AF50, SMC Co., Tokyo, Japan) and the flow rate was measured using a standard temperature and pressure corrected-thermal mass flow meter (Model: GFM57,Aalborg Instruments & Controls Inc., Orangeburg, NY, USA). Gas samples were then drawn to a dehumidifier, composed of CaSO4, and analyzed for CH4 in the non-dispersive infrared gas analyzer with a measuring range of 0 to 2,000 ppm and repeatability of 0.5% (Model: VA-3000, Horiba Stec Co., Kyoto, Japan) through a 6-mm pipe. The sampling pump (Columbus Instruments, Columbus, OH, USA) was used to enable this air flow.
The gas analyzer was calibrated 1 hour prior to the gas measurement. Zero gas of 99.99% N2 gas (Uniongas Co., Yongin, Korea) and span gas of 1,002 ppm (v/v) CH4(Uniongas Co.,Yongin, Korea) balancedwith N2 wereused for calibration.
Data acquisition unit
Air flow rate data measured at the thermal mass flow meter were transferred to the data logger (Columbus Instruments, Columbus, OH, USA) and were saved in the computer every 4 minutes. The CH4 concentrations in air samples were determined and saved in the computer every 10 seconds using software (Type: VA-3000, Horiba Stec Co., Kyoto, Japan).
Recovery test
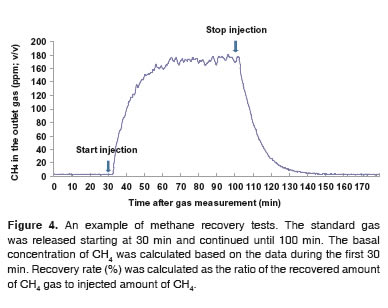
To validate the detection of CH4 gas in the chamber system, a recovery test was performed. In each test, the concentration of CH4 gas from the chamber was measured for approximately 180 min. Standard CH4 gas (1.67%, v/v) was released into the chamber at the rate of 1 L/min.
The gas release initiated at 30 min and terminated at 100 min. The basal concentration of CH4 from the chamber was estimated based on the CH4 gas emission from the chamber during the first 30 min. Based on the preliminary tests (data not shown), the concentration of CH4 returned to the basal concentration approximately 50 min after the termination of gas injection, and thus, 80 min of further gas analysis after stopping gas injection was assumed to be sufficient. Recovery rate (%) was calculated using recovered amount of CH4 gas and injected amount of CH4 gas.
Animal experiment
Four Korean native black goats with a mean body weight (BW) of 23.5 kg (SD = 1.0) were employed in the preliminary animal experiment. The goats were individually housed in each chamber and acclimated to the chamber before the initiation of animal experiment. A mixed feed was prepared using 50% of forages (2 to 5 cm cuts of tall fescue hay) and 50% concentrates (ground corn and soybean meal) on the basis of dry matter (DM).
The experiment lasted 10 days. The initial 6 days were an adaptation period to the feed, and during the following 4 days gas emission was measured and total feces and urine were collected. The feed was provided at amounts of 2% BW based on DM once a day at 1100 h. Water was sufficiently provided to last at least 24 hours. Shortly after feeding, the CH4 gas determination initiated and continued until 1.000 h of the next day.
Methane concentrations of each chamber were recorded for 4 min, and every 3 consecutive cycles of measuring gas in 4 chambers methane gas in reference air was measured for 4-min. In each 4 min measurement, first 3 min was regarded as a settling period and last 1 min was used for data calculation. Feces and urine excreted were collected and water intake was recorded between 1000 and 1100 h.
Feces were dried in an oven for 24 hours at 60 °C. Ingredient and fecal samples were ground using a micro hammer mill (Culatti AG, Zurich,
Switzerland) with a 1-mm screen. Samples were analyzed for DM by drying the samples for 2 hours at 135 °C (method 930.15; AOAC, 2005).
Results and Discussion
Recovery test
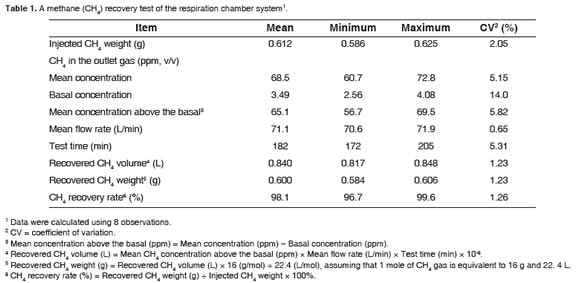
Values for recovery rate of CH4 gas ranged from 96.7 to 99.6% in 8 observations, and the mean value was 98.1% with the coefficient of variation (CV) of 1.3% (Table 1). These results demonstrated that the present system is working very accurately and precisely. In the other respiratory system by Klein and Wright (2006), recovery rate of CH4 gas was 101.1% but the variation was larger than in our test (CV = 4.5%; n = 6). The superior gas recovery rate of the present system compared with others may be attributed to the fact that this present system was designed for small ruminants, and thus, was smaller and than other systems designed for large animals. High accuracy of the gas analysis equipment may also be another positive contributor. An example of recovery test results was illustrated in figure 4.
Animal experiment
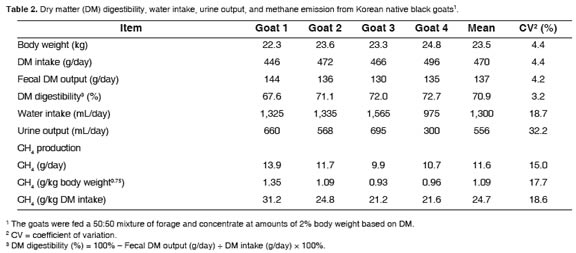
In the animal experiment, all goats remained healthy and consumed all the daily feed allowance. Dry matter digestibility was 70.9% in goats fed a 50:50 mixture of forage and concentrate at 2% BW (Table 2). In our previous experiment, DM digestibility was 68.0% (unpublished data) that is reasonably close to the present results. Islam et al. (2000) conducted an experiment using goats with a mean BW of 24.9 kg and provided 0.49 kg of feed daily. They reported that DM digestibility was 74.7% in goats fed a mixture of 50% Italian ryegrass and 50% corn. Shibata et al. (1992) reported that DM digestibility in goats fed forage and concentrate mixture with ratios of 70:30 and 30:70 was 64.9 and 72.6%, respectively. As DM digestibility can be affected by many factors, our dataappeared tobe within a reasonable range.
The CH4 gas productionwas 24.7 g/kg DM intake on average (Table 2). In a similar study conducted by Islam et al. (2000), a CH4 production of 23.5 g/kg DM intake was observed that was very comparable to our data. In other goat experiments, the CH4 gas emission varied from 16.2 to 24.6 g/kg DM intake when using various types of feed and feed intake (Shibata et al., 1992; Puchala et al., 2005; Animut et al., 2008). It seems that CH4 emission from goats may vary depending on many factors including feed type and feed intake.
The present novel respiration-metabolism chamber system is appropriate for measuring CH4 gas emission from small ruminants. The present system also enables nutrient digestibility determination and visual observation of animal behavior.
Acknowledgements
This work was funded by Rural Development Administration, Republic of Korea, and the project was a subunit of Agenda 5, "Development of Future Agricultural Technology to Adapt Climate Changes."
References
1. Animut G, Puchala R, Goetsch AL, Patra AK, Sahlu T, Varel VH, Wells J. Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Anim Feed SciTechnol2008; 144:212-227. [ Links ]
2. AOAC. Official Methods of Analysis. 18th ed. Association of official analytical chemists,Gaithersburg,MD. 2005. [ Links ]
3. Beauchemin KA, Kreuzer M, O'Mara F, McAllister TA. Nutritional management for enteric methane abatement: a review.Aust JExpAgric 2008; 48:21-27. [ Links ]
4. Beauchemin KA, McGinn SM. Methane emissions from feedlot cattlefed barleyor corndiets.JAnim Sci2005; 83:653-661. [ Links ]
5. Islam M,Abe H, Hayashi Y, Terada F. Effects of feeding Italian ryegrass with corn on rumen environment, nutrient digestibility, methane emission, and energy and nitrogen utilization at two intake levelsbygoats. SmallRuminant Res2000; 38:165-174. [ Links ]
6. Iwasaki K, Haryu T, Tano R, Terada F, Itoh M, Kameoka K. New animal metabolism facility especially the description of respirationalapparatus. BullNat InstAnim Ind1982; 39:41-78. [ Links ]
7. Klein L, Wright A-DG. Construction and operation of opencircuit methane chambers for small ruminants.Aust J ExpAgric 2006; 46:1257-1262. [ Links ]
8. Martin C, Rouel J, Jouany JP, Doreau M, Chilliard Y. Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil. JAnim Sci 2008; 86:2642-2650. [ Links ]
9. McGinn SM, Beauchemin KA, Coates T, Colombatto D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J Anim Sci 2004;82:3346-3356. [ Links ]
10. Moss AR, Givens DI, Garnsworthy PC. The effect of supplementing grass silage with barley on digestibility, in sacco degradability, rumen fermentation and methane production in sheep at two levels of intake. Anim Feed Sci Technol 1995; 55:9-33. [ Links ]
11. Puchala R, Min BR, Goetsch AL, Sahlu T. The effect of a condensed tannin-containing forage on methane emission by goats. JAnimSci 2005;83:182-186. [ Links ]
12. Shibata M, Terada F. Iwasaki K. Methane production in heifers, sheep, and goats consuming diets of various hay-concentrate ratios.AnimSciTechnol 1992;63:1221-1227. [ Links ]
13. Suzuki T, Phaowphaisal I, Pholsen P, Narmsilee R, Indramanee S, Nitipot P, Chaokaur A, Sommart K, Khotprom N, Panichpol V, Nishida T. In vivo nutritive value of Pangola grass (Digitaria Eriantha) hay by a novel indirect calorimeter with a ventilated hood inThailand. JpnAgricResQuart 2008; 42:123-129. [ Links ]
14. Swainson NM, Hoskin SO, Clark H, Lopez-Villalobos N. The effect of age on methane emissions from young, weaned red deer (Cervus elaphus) stags grazing perennial ryegrass (Lolium perenne)-based pasture. New Zeal J Agric Res 2007; 50:407-416. [ Links ]