What do we know about this problem?
Post-dural puncture headache is the most common complication in neuraxial anesthesia and analgesia techniques. The epidural blood patch is considered the standard of care for the management of this complication, but there are still a few controversial areas regarding its implementation.
What is the contribution by this article?
Over the past decade, regional techniques have been described that may offer other options under specific clinical conditions, that may and should be added to the therapeutic armamentarium but anesthesiologists are not well aware of these techniques.
INTRODUCTION
Post-dural puncture headache (PDPH) is the common complication in neuraxial anesthesia and analgesia techniques. 1 It may be the result of an accidental dural tear with an epidural puncture needle or following spinal anesthesia, particularly when using large gauge or sharp point needles. 2. The estimated incidence of accidental dural puncture with an epidural needle ranges between 50-80 % 2, whilst the estimated incidence of PDPH following spinal anesthesia may range between 4.2-11 %, depending on the type of needle used 3; though some publications report incidences as low as 1.5 % when using small gauge pencil-point needles. 4.
The severity of the symptoms and the resulting disability may lead to longer hospital stays and higher healthcare costs, in addition to patient dissatisfaction, particularly among the obstetric population, due to the limitation of the mother to take care of the newborn. 5,6 It should be highlighted that it is precisely the obstetric population that exhibits a higher risk of experiencing this complication because they are young female patients for whom neuraxial anesthesia and analgesia are the first choice. 2
DEFINITION
The International Headache Society included the following criteria in its definition of PDPH: develops within 5 days following the dural puncture, accompanied by neck stiffness and/or subjective hearing symptoms; additionally, there is a trend to resolve spontaneously in the next two weeks, or after placement of an autologous blood patch. 7 There are other associated symptoms and signs that may develop in more than 50% of the patients, including: worsening of the headache when standing up and improves in decubitus position, photophobia, diplopia and nausea; while the latter are not described as typical in the diagnostic criteria, nausea has been described as typical of this condition. 1,6,8 Always keep in mind that 5% of the obstetric patients may express atypical characteristics, and hence the postural sign may be absent 8
RISK FACTORS
The factors described in the literature are associated with the clinical and demographic history of the patient, and his/her physical characteristics, including: a previous history of PDPH or chronic headache, female gender, young age, low body mass index, neuraxial technique - sharp point needle, operator experience, time of the day when the procedure was conducted - and obstetric events - labor phase -7
PATHOPHYSIOLOGY
There are two major pathophysiological mechanisms in PDPH, that have implications in terms of the therapeutic options:
The cerebrospinal fluid (CSF) leak through the defect causes traction of the central nervous system structures, including the sensitive cranial nerves and results in pain.
Intracranial hypotension produces compensatory cerebral vasodilatation to maintain the intracranial volume and causes vascular type headache. 6
DIAGNOSIS
Usually the diagnosis is made based on the clinical presentation, using the above diagnostic criteria. If the patient develops any focal neurological sign or a persistent headache despite the general treatment measures, an interconsultation with neurology should be considered, in addition to diagnostic imaging such as contrast MRI. 7
The differential diagnosis, particularly in the obstetric population, includes tension headache or migraine, and even more severe conditions such as preeclampsia, meningitis, sagittal sinus thrombosis and subarachnoid hemorrhage. 9
TREATMENT
Several therapeutic options have been suggested to correct these alterations. Following is a description, emphasizing the new regional techniques, which may be feasible options in specific settings.
General measures
Notwithstanding the low level of evidence, most of these measures are still prescribed as first line approaches, and the anesthesiologist should be aware of the very low probability of the symptoms to improve spontaneously, particularly if the pain is severe.
It has been suggested that increasing the intake or the administration of IV fluids may increase the output of CSF; however, this measure has no evidence and has not proven to be effective. 10 The literature recommends maintaining a normal level of hydration and avoid dehydration. 5,8
In general, the treating physician recommends laying down in supine decubitus which seems a logical rather than a therapeutic measure, since due to the nature of the headache, rest improves the symptoms. However, it has not shown any positive effect on the treatment of PDHP 10 but on the contrary, prolonged rest - over 24 hours - may increase the risk of thromboembolic complications in the obstetric patient. 8
Pharmacological therapy
Caffeine is the most frequently prescribed drug to patients with PDPH, due its potential cerebral vasoconstrictive effect.
However, only two clinical controlled trials have assessed its effectiveness, showing benefit only during the first few hours. Additionally, the need to use the epidural blood patch was not avoided in the obstetric patients treated with caffeine, though the pain scored did improve, at least over the first few hours. 5,8 The oral or IV dose administered should be of 300 to 500 mg/day, with a maximum of 900 mg in 24 hours, to avoid adverse reactions, such as insomnia, anxiety, and even seizures, and preventing crossing into the maternal milk. 8 While in Colombia the IV presentation of this drug is not available and the available oral presentations are not enough to complete the recommended daily dose, taking one cup of coffee - which contains in average 50 to 100 mg of caffeine with an availability of almost 100% through this route represents a treatment option. 5
Conventional analgesics such as non-steroid anti-inflammatory agents and acetaminophen, are also routinely prescribed as part of the management approach, although their level of evidence for the treatment of this complication is low. 5 Whilst conventional analgesia does not relieve the etiology of PDPH, if the intensity of the symptoms warrants the prescription of more potent analgesics such as opioids, this should be done at least until a definitive control or treatment of the symptoms is achieved. 8
In contrast to the above-mentioned drugs, steroids and hydrocortisone in particular, have shown better results in clinical controlled trials. Their mechanism of action in this condition is unknown, but the effect is attributed to the action on the hypothalamic-pituitary-adrenal axis, which includes an increased production of CSF due to the active transport of sodium, the expansion of the blood volume and the release of aldosterone and increased production of endorphins in the brain. 5 The regimens suggested in the clinical trials recommend doses of 100 mg every 8 hours for 48 hours.
Sumatriptan (a serotonin receptor agonist) causes cerebral vasoconstriction and helps with migraine-type headache, but there is no evidence about its efficacy in PDPH. 7
Epidural blood patch
This technique is based on injecting autologous blood into the epidural space, through a needle, in order to create a mechanical sealant effect on the existing dural defect. Most patients experience immediate symptom relief, which leads to the conclusion that the increased lumbar canal volume immediately increases the CSF pressure.
Many aspects about this technique have been controversial in the literature, including the optimal timing, the volume and the substance to be injected, inter alia.
This therapeutic option, though invasive, is the approach with the best evidence in terms of improving pain and duration over time 2,6; hence, it should always be considered part of the management algorithm. When the conservative approaches fail and severe pain persists, the epidural blood patch should be considered, since inadequate and delayed PDPH therapy may have serious short and long term consequences, such as venous sinus thrombosis, subdural hematoma, hearing loss, paralysis of the cranial nerves and chronic headache. 6,11 Just as with any medical procedure the risk and benefits should be clearly explained to the patient, since the success rate is only around 70 %. 12 Emphasis should be made in the potential need repeat the procedure for total symptom relief.
Injection of crystalloids into the epidural space has been assessed as a prophylactic and therapeutic measure for PDPH, either in bolus or as a continuous infusion. The increased spinal canal pressure may result in transient headache relief, but the downside is that these substances are rapidly absorbed from the epidural space and fail to result in sustained improvement. 8,13 Several colloids such as dextran, starch and gelatins have been assessed in small series of cases for injection into the epidural space; however, there are no controlled clinical trials to ascertain their safety and therefore their routine use is not recommended. 8 Another approach described in the literature is the use of epidural morphine; but although some trials report improvement in PDPH, it may increase the incidence of nausea and vomiting. Moreover, a potential increased risk of respiratory depression has been mentioned, because of the potential of the opioid crossing into the intrathecal space, through the dural defect.
Some authors have researched the use of the epidural blood patch as prophylaxis; this means, through the epidural catheter, before its removal and after the occurrence of an accidental dural tear. The studies to assess this approach exhibit several design flaws and hence the evidence is not conclusive. The prophylactic use of the epidural blood patch is therefore nor recommended for the time being. 6
The relief of symptoms using the blood patch may be explained through several mechanisms: first, the sealant effect of the blood plug on the dural orifice prevents the ongoing CSF leak and generates an inflammatory reaction that also contributes to close the defect. However, the improvement of the headache is in most cases immediate and this is not explained only via this mechanism, since the CSF volume reestablishes at a rate of 0.5 ml/hr. Second, the increased pressure inside the spinal canal when transferred into the intracranial space, causes cerebral vasoconstriction, and hence offset the mechanisms causing the headache. 6,12
The perfect timing for an epidural blood patch has also been controversial. Some retrospective trials have suggested that the efficacy of the patch drops if it is done during the first 48 hours following the puncture, and patients should be warned about this. The suggestion is then that is the symptoms are not too severe, and there are no alarm signals, conservative management should be administered for a sensible period of time, since the conditions tends to improve spontaneously, particularly when the PDPH is the result of a spinal puncture. Moreover, if the symptoms are severe and may be attributed to a large dural defect, the epidural blood patch should be an early therapeutic approach, since the likelihood of improving with conservative measures is poor and the risk of serious complications is higher. 12 Nonetheless, this early approach may unfortunately increase the risk of having to repeat the procedure to achieve complete improvement of symptoms.
The puncture site to do the patch should be the same where the initial puncture was performed or one level below, since the injection of contrast medium and the study of the contrast spread into the epidural space, have shown that the injected fluid spreads mostly in a cephalic direction. 12 In terms of the blood volume injected, the literature suggests that the optimum amount ranges between 15 and 20 cm3, and above this volume the risks may exceed the benefits. If the patient experiences lumbar pain during the injection, it must be discontinued. 1,6,12 If despite placing a second blood patch the headache relapses, the diagnosis should be reconsidered. 7
Some of the complications of the epidural blood patch described in the literature include back ache, though usually it is self-limited. Mention has been made of the development of facial paralysis, permanent spastic paraparesis, cauda equina syndrome and meningitis, though the latter is usually associated with a poor aseptic technique or to the dissemination of a systemic infection.
The risk of administering blood to cancer patients or patients with a viral infection is still a concern. The recommendations in the literature focus on avoiding placing the blood patch because of the risk of injecting infected blood into the central nervous system and only use it in severe cases that respond poorly to conservative measures.
Finally, there has been some controversy around the advisability of advancing a spinal catheter through the orifice created after an accidental dural puncture to be left between 12 to 24 hours after the puncture. Similar to the previous discussion, the evidence is controversial. Some authors have pointed to the lack of high quality clinical controlled trials that enable a clear conclusion about this issue. In 2010, Bradbury et al said that the existing literature was far from proving that this practice was of any real use. 14
A meta-analysis published in 2013 suggested a reduction in the need to use the epidural blood patch in obstetric patients with a spinal catheter; however, no reduction in the incidence of PDPH was actually shown. 6 While this procedure could impact the mechanisms leading to PDPH, generating an inflammatory reaction around the continuity solution and avoiding a new puncture to relocate the epidural catheter, the risks and benefits should always be balanced. This practice should not be adopted in centers without an adequate protocol for obstetric analgesia, since any mistakes in the administration of the dose and the volume of drugs may lead to serious and potentially fatal complications.
Regional techniques as an option to the epidural blood patch
There are some clinical scenarios in which the patient may refuse treatment with an epidural blood patch (for example Jehovah's witnesses), or they may be a contraindication for its use. Less invasive techniques may be offered to the patient when the PDPH is moderate to low intensity. 5,15.
Greater occipital nerve block
The greater occipital nerve block (GONB) is a superficial block that can be done under ultrasound guidance at the patient's bedside. It has been frequently used for managing some specific types of chronic headache, but some series of cases have been recently published showing its benefit in the management of PDPH. 15-17
The greater occipital nerve originates at the medial root of the dorsal branch of C2. It ascends after emerging through the suboccipital triangle, below the major oblique muscle of the head and becomes superficial between 2.5 and 5 cm inferolateral to the occipital protuberance. It innervates the skin, the muscles and vessels of the scalp, all the way to the vertex where its starts sharing territory with the ophthalmic branch of the trigeminal nerve.5,18
Blocking the greater occipital nerve in the presence of PDPH helps by a neuromodulation effect and decreased central sensitivity resulting from the irritation of the meninges and the paraspinal muscles, blocking the afferent fibers to the dorsal horn of the spinal cord. Additionally, the sensitive neurons of the upper cervical cord are close to the trigeminal caudal nucleus, and hence its afferences may also be blocked with this technique. 18
An ultrasound guided block requires a high-frequency lineal transducer, preferably a needle to block the peripheral nerve, 0.5% bupivacaine with epinephrine 1:200.000: 3-5 ml as local anesthetic agent, and 1 mg of dexamethasone; steroid use is recommended to prolong the duration of the block. The use of hypodermic needles is also described in the literature to administer the block, since these needles can be visualized easily under ultrasound and the risk of neurological damage with this block is minimal; moreover, these needles are less expensive than a block needle. The approach of the needle may be in-plane or out-of-plane, although the latter approach minimizes the distance to be covered.
With the patient in a prone position or sitting down, a scan is performed with the probe in the axial position, starting from the occiput, where a hyperechoic line with acoustic shade is identified, corresponding to the occipital bone. Sliding the probe caudally, the posterior arch of the C1 vertebra is identified, and more caudally the posterior arch of the C2 vertebra. 19 The medial aspect of the probe is fixed at this point, rotating the lateral side in a cephalic direction (for a diagonal orientation), that renders the image of a longitudinal axis of the obliquus capitis inferior inserting into the C1 and C2 (Figure 1).
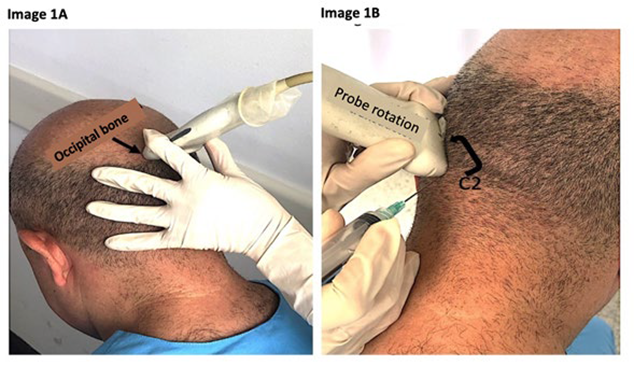
A. Placing the probe at the level of the occipital bone and move downwards to the spinous process of C2. B. Cephalic lateral rotation. SOURCE: Authors.
FIGURE 1 Probe placement for greater occipital nerve block.
The plane between the obliquus capitis inferior muscle, which the one running deeper in the image, and the immediately superficial, the splenium, is where the local anesthetic is deposited. 19
Another option could be localizing the occipital artery which is 1-2 cm inferior and lateral to the occipital protuberance and administer the injection immediately medial to the protuberance 18(Figure 2).
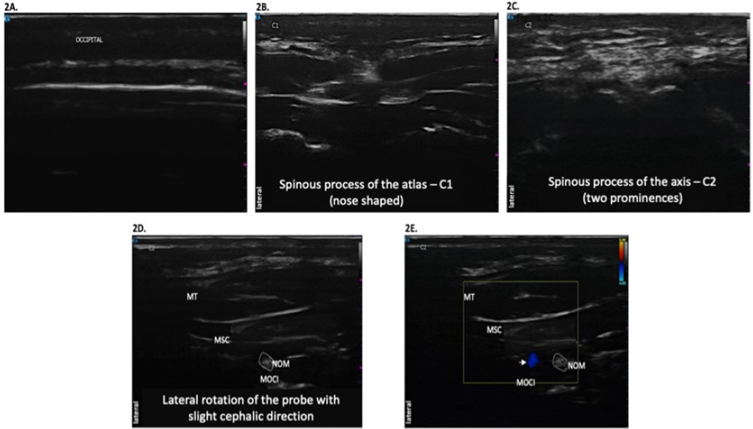
C1 is identified (nose-shaped). C. Movingon caudally, the spinous process in C2 is identified, with two prominences. D. The probe is rotated laterally with slight medial orientation to identify the three muscle layers (from superficial to profound): trapezius (MT), semispinous of the head and the obliquus capitis inferior muscle. The greater occipital nerve (NOM) is in the middle of the last two. E. Doppler color mode localizing the greater occipital artery (horizontal arrow). SOURCE: Authors.
FIGURE 2 Localization of the greater occipital nerve.
The evidence of this block for treating PDPH is still poor. One of the largest case reports has 21 patients, describing significantly improved pain scores in the visual analogue scale (VAS) in patients with initial assessment between 4 and 6. For patients with a higher initial VAS score, the block reduced the pain scores, but the symptoms tended to relapse. 16
A retrospective study was recently published where 42 obstetric patients with PDPH received blocks (GONB and/or sphenopalatine). In the end, only 9 patients required an epidural blood patch because of symptoms relapse. All patients had experienced accidental dural punctures with Tuohy needles. The author suggest then that the blocks may be particularly useful when the dural defect is not too large and therefor the CSF leak is not significant. 15
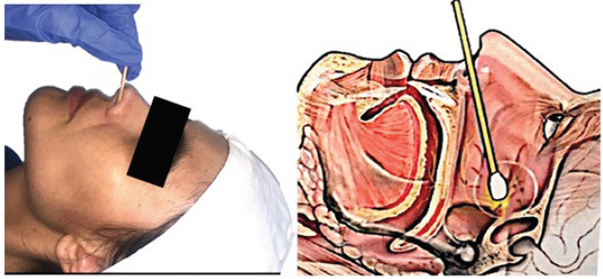
Sphenopalatine ganglion block
The second regional technique that may be used as an option is the sphenopalatine nerve block. This ganglion contains parasympathetic and somatosensory fibers and is localized in the pterygopalatine fossa, posterior to the mid turbinate. 5,19 One of the approached is through the nose and the benefit of its block in case of PDPH is due to the vasoconstriction resulting from the parasympathetic block. 20 Moreover, due to its relationship to the fifth cranial nerve (trigeminal nerve), there may be a simultaneous relief of the frontal headache.
This is an easy block and requires minimal training. With the patient in supine decubitus and the neck slightly extended, a long swab impregnated with local anesthetic is inserted through each of the nasal fossae, until hitting the nasopharyngeal wall. 20 Some case reports have used 2 or 4 % lidocaine in gel or in liquid form, and other use 0.5 % bupivacaine. 21 These swabs should be left in place for at least 10-15 minutes. The procedure may be repeated in order to ensure an adequate block (Figure 3).
There are no clinical controlled trials assessing this block so far. The available evidence is based on retrospective case series which have shown improvements in the VAS scores and a reduced number of patients requiring an epidural blood patch. 20
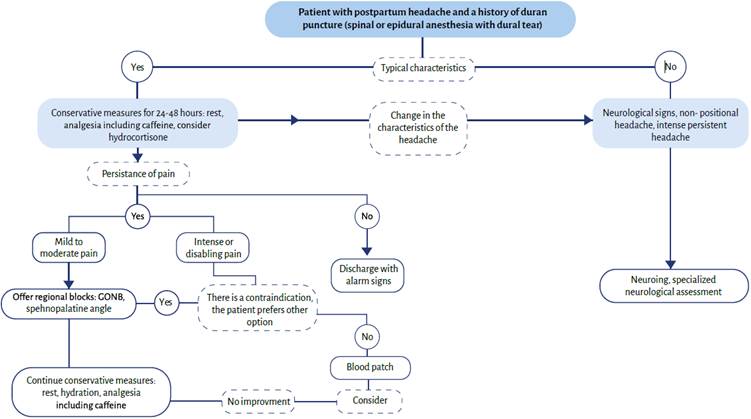
Based on the literature review conducted to write this article, and based on the experience of the authors, they suggest the following therapeutic algorithm (Figure 4). This is just a suggestion and is not based on a systematic review of the evidence.
CONCLUSIONS
PDPH is a common and debilitating complication that deserves the attention and follow-up of the anesthesiologist. Conservative therapy, while administered in most cases, has little evidence and usually is not very helpful in severe cases. The possibility to use a blood patch should always be part of the management algorithm, since there are serious and potentially fatal complications derived from a delayed and inadequate management of this condition. In the last few decades, regional techniques have been described that could be applicable in non-severe PDPH, with no alarm signs and when the epidural blood patch is contraindicated.











 text in
text in