What do we know about this issue?
Surface electromyography (EMG) is a non-invasive and simple procedure with the potential for high sensitivity. It is painless and reduces the risk of invasive complications, thus promoting well-being, comfort, and cost-effectiveness in the care of patients with respiratory disorders compared to other methods.
Reference values and data acquisition techniques, as well as the equipment used for this purpose, are not yet standardized or comparable among them.
¿Qué aporta este estudio de nuevo?
This study carries out an initial examination of diaphragmatic function using EMGs in healthy individuals, aiming to contribute to the standardization of methods and physiological values for this variable.
INTRODUCTION
The diaphragm is the main inspiratory muscle 1,2 and it is involved in modulating the intraabdominal pressure, postural stability, cardiac function, lymph flow, labor, swallowing, and emesis; additionally, it provides a barrier against gastroesophageal reflux, among other physiological processes. 1 The assessment of this muscle is clinically important because several pathological conditions result in diaphragmatic weakness, paralysis and fatigue, that could be irreversible. 3 The most frequent alterations are diaphragmatic dysfunction, diaphragmatic paralysis, or structural changes such as congenital agenesis, congenital duplication, eventration, diaphragmatic hernia, inter alia. 3,4
The assessment of the diaphragm may be conducted using invasive and non-invasive methods, and biological markers. 5-8 These methods may be helpful to predict respiratory function, as adjuvants when withdrawing mechanical ventilation, or in cardiopulmonary rehabilitation. Some of the most common methods are diaphragmatic excursion 8, estimating the transdiaphragmatic pressure with esophageal and gastric balloons 5, transdiaphragmatic ultrasound 9, and the measurement of skeletal troponin 1 (sTnl) 8; however, many of these methods are invasive, painful and observer-dependent.
ln contrast, the diaphragmatic surface electromyography (EMG) is a simple, non-invasive, highly sensitive procedure 10, painless and reduces any potential invasive complications. If properly standardized, this approach could promote wellbeing, comfort and cost-effectiveness in patient care, as compared to other methods. However, there are no accurate data in healthy individuals, neither in patients with respiratory pathologies to establish reference values; moreover, the techniques to capture data and the equipment used are not yet comparable. 8,11 Among other design methodologies, there is a need to develop a baseline of physiological, exploratory and longitudinal studies, in different groups of individuals according to age, respiratory and physical conditions. The primary goal is to generate parameters that could provide the foundation for future studies, particularly adapting the technique and adjusting the monitors to capture signals, as well as the parameters for procedures to filter cardiac electrophysiological signals. Consequently, this study is intended to do an initial exploration in healthy individuals. 12
The purpose of the study was to assess and describe the diaphragmatic function using EMG in a healthy population between 18 to 40 years old, based on their physical characteristics such as age, gender, and a few anthropometric variables, in order to contribute to standardize the methods and the physiological values for this variable.
METHODS
An observational, exploratory, cross-sectional study was conducted in individuals with no comorbidities, aged between 18 and 40 years. 4,13 The age range was selected taking into consideration the findings by various authors who identified that in average, sarcopenia starts to develop after 40 years of age. 4,14,15
The sample included 50 individuals and it was estimated according to a formula of confidence intervals for medians based on the standard deviation derived from the study by oliveira da Silva et al., who found a root mean square (RMS) SD = 17.88 μV of the RMS (μV) for healthy subjects in the Epidat 3.1 software. 16. Individuals with any contraindication for the EMG, with pacemakers or cardio defibrillator, with a history of recent surgeries or diaphragmatic lesions, who had received mechanical ventilation during the past 48 hours regardless of the modality, a history of corticosteroid use or muscle relaxants in the past two weeks, and individuals with underlying neurological, musculoskeletal, neuromuscular, respiratory and cardiovascular pathologies that could interfere with the results were excluded.
This project was assessed and approved by the ethics committee of Fundación Universitaria Navarra, minutes 007-1, of June 4, 2020, and all of the participants signed an informed consent before being admitted to the study.
The measurement of electrical potentials resulting from muscle contraction was done with EMG 10. The motor unit action potentials were recorded, together with the frequency or pressure variations per unit of time (1 second) in the EMG signal which ranges between 2-500 Hz, in addition to amplitude variations, equivalent to energy changes of the signal generated during the passage of a wave which is around 2.1 microvolts (μV) for large muscles, with a frequency of 500 Hz and a sensitivity signal amplitude of 500 μV for the diaphragm and a frequency of 300 Hz and sensitivity signal amplitude of 300 μV for other muscles, such as the abdominal muscles. 17-20 The electronic circuit measured was assessed in a portable computer to record the EMG, using the Power Lab® hardware and Labchart® software with the registered numbers shown in the RMS. 16
The volunteers were asked to remain in supine position with a 35° head elevation and the leads arrangement was matrix/line (3M®), two adhesive leads in the paraxiphoid region, 5 cm below de xiphoid process and other two in the costal margin region, bilateral, at a 16 cm distance 10. Posteriorly, the respiratory band was positioned approximately on the sixth intercostal space to record the ventilatory frequency and depth. Based on the equipment management protocol, the recording was initiated in 5 Labchart® channels (Figure 1): oxygen saturation was recorded in channel 1; respiratory movements in channel 2 -the respiratory movements measured through the respiratory band -; the thoracic electrical signal was registered in channel 3, including the respiratory activity signals and cardiac activity; channel 4 was used for the first filtered tracing of the electrical activity of the diaphragm and respiratory muscles with a 1200 Hz Notch type filter; and channel 5 was used to filter channel 4 with a 500 Hz band filter. The signals were filtered so that the respiratory activity was the dominant activity for study purposes, minimizing the cardiac activity wave as much as possible, managing the high and low frequency sections expressed in Hz.
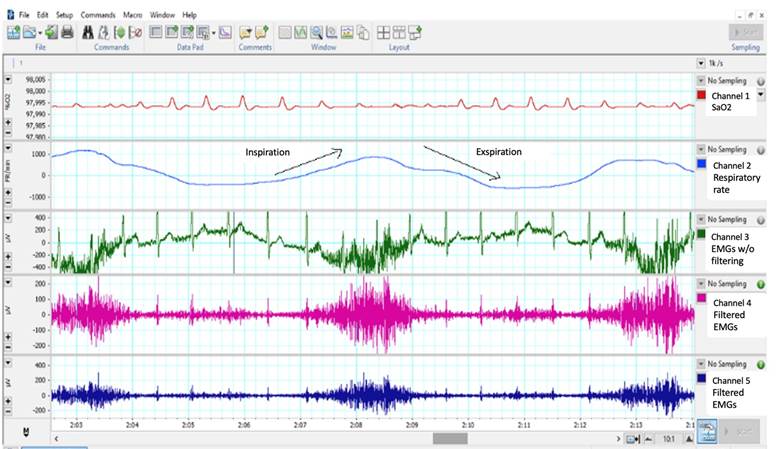
Channel 1: SaO2 of the participants. Channel 2: Recording of the respiratory band positioned in the chest. Channel 3: Electrical activity of the diaphragm, other respiratory muscles and the heart during three forced breathing cycles. Channel 4: The first tracing is the filtered electrical activity of the diaphragm and respiratory muscles with a 1200 Hz Notch type filter. Channel 5: Filter channel 4 with a 500 Hz band filter. Source: Authors.
Figure 1 Values and measurement channels of the diaphragmatic surface electromyography for vital capacity and tidal volume.
Then the patient was instructed to breath normally for 2 minutes, in order to be able to record the tidal volume; then the patient was asked to take 3 forced inspirations and expirations (Figure 1).
Statistical analysis
The discrete or nominal variables were expressed in frequencies and proportions. Central tendency and scatter were used for the continuous variables and the Shapiro-Wilk for normality test was used to establish the behavior of the continuous variables. Correlation measures were taken (Spearman coefficient to determine the correlation of the relevant variables). The statistical test used to analyze the measurement differences was the Mann-Whitney U test, and for differences in proportion, the Fisher's test. A 5% level of significance was accepted for all the analyses (a = 0.05) and the differences were described together with 95% confidence intervals. The statistical analysis was conducted using the epi R software.
RESULTS
This study included 50 participants: 22 women (44 %) and 28 men (56 %), with a mean age of 23.3 years, (SD ± 4.5) for females, and 27.1 years for males (SD ± 6,4) (Table 1). The average weight for females was 59.3 kg (SD ± 11.39), and for males 82.3 kg (SD ± 16.1). The detailed characteristics of the participants are illustrated in Table 1.
Table 1 General characteristics of the participants in the study (n = 50).
| Variable | Females (n = 22) Mean ± SD n (%) | Males (n = 28) Mean ± SD n (%) |
|---|---|---|
| Age (years) | 23.3 ± 45 | 27.1 ± 6.4 |
| Height (cm) | 163 ± 5.4 | 174 ± 7.6 |
| Weight (kg) | 59 3 ± 11.3 | 82.3 ± 16.1 |
| Body surface (m2) | 1.62 ± 0.16 | 1.93 ± 0.18 |
| Body mass index (kg/m2) | 22.2 ± 4.1 | 27 ± 4 |
| Low weight | 3 (13) | 1 (4) |
| Normal weight | 16 (73) | 7 (25) |
| Overweight | 2 (9) | 57.14 (16) |
| Obesity I | 0 | 2 (7) |
| Obesity II | 1 (5) | 2 (7) |
| Physical exercise > 150 min/week | 11 (50) | 15 (54) |
| Physical exercise < 150 min/week | 11 (50) | 13 (46) |
SD: standard deviation.
Source: Authors.
The RSM for females was 13.94 μV (SD ± 4.05 μV). For males, the mean value obtained was 13.31 μV (SD ± 5.48 μV) (Table 2).
Table 2 Root Mean Square Results (RMS) for tidal volume and vital capacity per 2 minutes.
| Tidal volume | Females | Males |
|---|---|---|
| Mean μV (95 % Cl) |
13.94 (12.1-15.7) |
13.31 (11.17-15.43) |
| SD μV | 4.05 | 5.48 |
| P25 μV | 9.92 | 10.22 |
| P50 μV | 15.28 | 13.29 |
| P75 μV | 17.14 | 16.11 |
| P90 μV | 17.57 | 19.27 |
| P95 μV | 18.99 | 24.65 |
| Vital capacity | Females | Males |
| Mean μV (95 % CI) |
22.4 (18.9-25.9) |
23.24 (19.9-26.56) |
| DS μV | 7.9 | 8.58 |
| P25 μV | 16.73 | 16.53 |
| P50 μV | 20.51 | 22.52 |
| P75 μV | 28.23 | 28.47 |
| P90 μV | 35.34 | 33.73 |
| P95 μV | 37.61 | 41.5 |
μV: Microvolts; SD: Standard deviation.
Source: Authors.
The RMS measurement during three consecutive breaths of vital capacity, resulted in higher values for males - 23.24 μV (SD ± 8.58). Anthropometric variables were also compared, including height, weight and BMI, among other characteristics such as age and total body surface (TBS); with the RMS at VT, VC and R1, R2 and R3, a correlation was found between weight and RMS R1, and also with TBS (Table 3).
Table 3 Correlation between the physical characteristics of the participants and RMS VT, RMS VC average and in R1, R2 and R3.
| Variable | RMS VT | RMS CV | RMS R1 | RMS R2 | RMS R3 |
|---|---|---|---|---|---|
| Age | Rho = 0.209 p = 0.144 | Rho =0.188 p =0.190 | Rho =0.129 p =0.370 | Rho =0.070 p = 0.629 | Rho = 0.109 p = 0.448 |
| Height | R2 =0.141 p = 0.232 ** | Rho = 0.160 p = 0.265 | Rho = 0.224 p = 0.116 | Rho = 0.129 p = 0.371 | Rho = 0.160 p = 0.266 |
| Weight | Rho = 0.129 p =0.369 | R2 =0.246 p =0.084 | Rho =0.298 p =0.035 | Rho = 0.154 p =0.284 | Rho = 0.181 p = 0.206 |
| BMI | Rho =0.07 p =0.585 | Rho =0.227 p =0. 112 | Rho =0.246 p =0.084 | Rho = 0.113 p = 0.430 | Rho = 0.162 p = 0.258 |
| TBS | Rho = 0.129 p = 0.369 | Rho =0.246 p = 0.084 | Rho = 0.298 p =0.035 | Rho = 0.154 p = 0.284 | Rho = 0.181 p = 0.206 |
Spearman coefficient, ** Pearson coefficient; Ri: Breath one; R2: Breath two; R3: Breath three.
Source: Authors.
Additionally, the BMI categories and the different RMS measurements were compared, and no relevant differences were identified. A stratification based on age and the RMS measurements for each range was performed, and 52 % of the sample was within an age range of 18 to 21 years. The highest RMS mean in VT breaths corresponds to the age range between 37 to 39 years (20.01 μV). On the other hand, the lowest RMS VT value was found in the 2730 year old group (6.52 μV). In contrast, the lowest RMS VC value was found in the 30-33 year old group (19.95 μV). In general for all age ranges, the RMS standard deviation did not show very scattered values, except for the 27-30 year old range (SD ± 23.4).
DISCUSSION
This study assessed the diaphragmatic function using EMG to contribute to the RMS reference values in young adults 18 to 40 years old. Several body characteristics were also analyzed, including sex, age, TBS, BMI and physical condition. The EMG detected and analyzed the electrical activity of the diaphragm muscle associated with the contractile and motor activity - both voluntary and spontaneous. 21
A few differences were found based on the body characteristics of the subjects studied, which were in some cases consistent with the available literature, and other cases were contradictory. Oliveira da Silva et al. 16 studied the differences in the electrical activity of the diaphragm in two groups (healthy individuals and patients with liver disease), including subjects with similar body weights and physical characteristics, with older ages than the subjects in this study. The RMS for healthy subjects was 49.39 μV, (SD ± 17.88), while for liver disease patients the RMS was 56.56 μV (SD ± 34.64); the latter could have sarcopenia, which develops after age 40, in addition to underlying conditions related to age, changes in the nutritional and metabolic status, which have an impact on the muscle dynamics. Oliveira da Silva et al. concluded that higher RMS values in patients with liver disease were associated with higher ventilatory effort 4,16,22; though they agreed that this instrument may be helpful as a tool in respiratory monitoring.
In this study the average RMS values were lower than those reported by Oliveira da Silva et al.: 22.4 μV for females and 23.24 μV for males; in VC ventilation, the highest mean RMS of VT and VC was for an age range between 37 to 39 years, but just 6 % of the sample was within this range. Likewise, for the lowest RMS value in VT for the 2730 years age group, which represented 4 % of the study population. These conflicting results may be associated with differences in age and body composition of the participants in both trials. 16 However, Duarte et al. (10) found that the RMS values of the right and left diaphragm in spontaneous mode with minimal ventilation parameters, were 26.68 ± 10.92 μV and 26.55 ± 10.53 μV, respectively; and the RMS values after extubation were 31.93 ± 18.69 μV to 34.62 ± 13.55 μV, which is very similar to the findings in this study. 10
Other studies such as Hawkes et al., examined healthy individuals and considered the effect of adding inspiratory load on the electrical activity of the diaphragm. 23 They found an increase in the RMS values during maximum inspiratory pressure, associated with the increased work of breathing during forced inspirations (maximum inspiratory pressure: 125.6 ± 30.8 cm H2O). The physical characteristics and the age of the subjects in the study by Hawkes et al. were similar to those included in this study, as well as the reported RMS values. 23. Moreover, other analyses such as Lokin et al. showed that the diaphragmatic EMG and the transesophageal EMG had a time correlation and concordance. With optimization of signal stability, diaphragmatic EMG may become a useful respiratory monitoring tool, although there is a need for longitudinal studies of different populations to fine-tune the technique and reference values. 24,25 Currently there are clear guidelines for electromyography methods for various muscles 17; however, the respiratory muscle bundle remains a challenge due to its function and anatomical location.
This research project also explored some correlations with regards to the physical characteristics described as relevant by other authors; a correlation was identified between the TBS and higher RMS values; since 72.7 % of the female participants had an adequate BMI and 57.14 % of males were overweight, the RMS values were observed to be higher in individuals with a higher BMI. The is an increasing trend in the RMS values when the BMI is higher, which is consistent with the literature. 26-28 The study by Steier et al. used transesophageal electromyography with multipolar electrode and they reported that the higher the BMI, the work of breathing increases 27; this could be associated with thoracic changes in obese individuals which are reflected in increased elastic resistance, both of the lung and the chest wall, increasing the respiratory effort due to mechanical overload. Moreover, it was associated with a decrease in the pulmonary volumes affecting the inspiratory capacity, the expiratory reserve volume and VC. 28
Additionally, some cases of fat infiltration in the respiratory muscles have been described, which are accompanied by muscle dysfunction, increased diaphragmatic electromyography activity, which does not necessarily involve increased inspiratory muscle pressure or insufficient muscle contraction. 28 According to Kelly et al., obesity decreases the chest compliance by up to 65 %, because the excess adipose tissue in the chest and abdomen hinder the chest expansion. 29 Moreover, people with obesity as opposed to normal weight individuals, generate higher VT inspiratory pressures to offset their poor respiratory performance which results in increased work of breathing; hence this population develops increased strength and capacity of the respiratory muscles. This may explain the increased RMS in overweight and obese individuals 29.
The RMS behavior with regards to gender showed no differences in this study. However, the measurements were taken at rest and some studies have found differences during physical activity. Molgat-Seon et al. found higher diaphragmatic RMS values in women and elderly adults during exercise. 30 Additionally, Mitchell et al. also assessed EMG of the diaphragm and extra-diaphragmatic inspiratory muscles during exercise and they identified gender differences and reported that extra-diaphragmatic inspiratory muscles RMS was higher at all times during submaximal exercise in females. 31 They concluded that there may be a greater reliance on the extra-diaphragmatic inspiratory muscles in women relative to men. 31
These sex differences were also reported by Guenette et al., who suggest that muscle fatigue in women may be lower because during exercise the load is distributed more uniformly over the respiratory muscles and there is also the involvement of the extra-diaphragmatic inspiratory muscles thus reducing the load on the diaphragm. This phenomenon has been associated with less muscle mass in women giving rise to the need to distribute the load on several respiratory muscles. 32,33. Moreover, Hawkes et al. 23 identified some sex differences, but these were not conclusive. 2,34
The participants who exercised regularly >150 minutes per week, had lower VT RMS values as compared to those with a relatively sedentary lifestyle. This may be associated with overweight or decreased muscle mass since weight increase leads to higher respiratory effort; in contrast, physical activity has a favorable muscle effect. 3
With regards to the value for monitoring the respiratory function, Hernández-Valdivieso et al. assessed the relationship between positive end-expiratory pressure (PEEP) on the respiratory muscle activity using EMG in individuals with non-invasive mechanical ventilation and obtained values associated with muscle work during inspiration and expiration. They concluded that EMG is quantitatively related to the PEEP level and the change in diaphragmatic and sternocleidomastoid activity. 11,20,35,36 This could be valuable in bedside monitoring of the critically ill patient; however, it is still necessary to further standardize the reference values and the specific signal filtering technique for these muscle groups in order to make them comparable between patients.
This research contributes with exploratory physiological data that may be the basis for further studies on a future instrument for bedside monitoring of the respiratory muscle function, once the above-mentioned challenges are overcome. The main limitations were the absence of complete spirometry measurements to correlate the EMG results with the RMS; additionally, no measurements were taken of the frequency domain of the electromyography waves and the sample was small. Moreover, there is a gap in our knowledge about the reference values for standardizing the diaphragmatic EMG amplitude during maximum voluntary isometric contractions and during moderate to intense physical activity. The findings in these young healthy subjects may not be generalized to other populations such as the elderly, critically ill patients or patients with respiratory failure.
CONCLUSIONS
The diaphragmatic function was assessed with the use of EMG in a population of healthy individuals, 18 to 40 years old. The tidal volume RMS for two minutes was 13.94 μV in females and 13.31 μV in males, while the vital capacity was 23.24 μV for males and 22.4 μV for females.
ETHICAL DISCLOSURES
Ethics committee approval
The study was approved by the Ethics Committee of Fundación Universitaria Navarra, minutes 007-1, of June 4, 2020
Protection of humans and animals
The authors declare that no experiments were conducted in humans or in animals for this research. The authors declare that the procedures followed were consistent with the ethical standards of the committee on responsible human experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
ACKNOWLEDGEMENTS
Contribution by the authors
CYRT and CMSS: Study planning, data collection, interpretation of the results and drafting of the manuscript.
MEMP: Study planning, data collection, interpretation of the results, analysis of the data and drafting of the manuscript.
AMZ: Data collection, drafting of the manuscript.











 text in
text in 



