INTRODUCTION
Leaf anatomy provides important information about aspects of plant physiology. Thus, anatomy techniques are important tools to link morphological and physiological traits of plants (Brown, 1958; Hattersley and Watson, 1976). In studies about transition between the C3 and C4 pathways, leaf anatomy characterization is the first step in order to determine transverse sectional characteristics of the mesophyll (M) and the bundle sheath (BS) cells, and the degree of approximation between these two compartments (Dengler et al., 1994; Khoshravesh et al., 2016; Lundgren et al., 2019). The C4 via ofthe photosynthesis is an evolutionary novelty derived from C3 pathway, in response to the increase of photorespiratory activity (Sage, 2016). As most of the C4 plants have a spatial separation, where the initial CO2 fixation occurs in M cell and the decarboxylation in the BS cell, there is a CO2 concentration mechanism around the Rubisco site, avoiding its oxygenase activity (Sage et al., 2011; Sage et al., 2014).
It is well documented that first steps for C4 evolution includes anatomical and ultrastructural modifications to establish the Kranz anatomy (Gowik et al., 2011). Thus, a reduction in the distance between two vascular bundles for the metabolite shuttle and an increase in the number of organelles in the bundle sheath cells for the photosynthetic activity are expected (Lundgren et al., 2019). Interestingly, some C3 species have a leaf anatomy closely related to C4 plants, especially in Poaceae family (Lundgren et al., 2014). In these cases, a large investment in BS tissue, which requires just few modifications in order to set C4 pathway, is observed (Christin et al., 2013; Lundgren et al., 2014).
During the evolution of photosynthesis, C4 pathway has evolved independently more than 60 times in angiosperms (Sage, 2016). Monocots account the highest number of C4 species (Sage et al., 2011), especially in the Poaceae family, which has the largest number of C4 transitions, about 20 times (Christin et al., 2012). All C4 grasses are clustered in PACMAD clade (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae, and Danthonioideae), showing that this clade probable has some characteristic to facilitate this evolution (Christin et al., 2010; GPWG II, 2012). Of all C4 origins in PACMAD, 15 occurred in the subfamily Panicoideae, its greatest lineage, which includes the subtribe Arthropogoninae (Giussani et al., 2001; Edwards and Smith, 2010; GPWG II, 2012).
The subtribe Arthropogoninae has seven genera with C3 photosynthesis and nine genera with C4 photosynthesis, type NADP-ME (Morrone et al., 2012). Among them, the genus Apochloa Zuloaga & Morrone is described as a C3 one, and it is phylogenetically related with two C4 genera: Coleataenia Griseb. and Cyphonanthus Zuloaga & Morrone (GPWG II, 2012; Morrone et al., 2012). Considering the modifications that occur in C3 grasses related to C4 genera, it is important to determine whether Apochloa species present anatomical traits that can be considered a pre-disposition to the C4 pathway. Thus, the aim of this paper was to describe leaf anatomy by means of transverse sections of four Apochloa species in order to investigate if the genus has characteristics that facilitate the C4 evolution in the subtribe Arthropogoninae.
MATERIAL AND METHODS
Site description and plant material
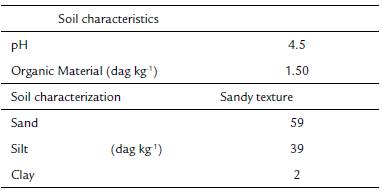
Anatomical studies were carried out with four Apochloa species: A. euprepes (Renvoize) Zuloaga & Morrone, A. lorea (Trin.) Zuloaga & Morrone, A. molinioides (Trin.) Zuloaga & Morrone, and A. poliophylla (Renvoize & Zuloaga) Zuloaga & Morrone. Species were collected in February 2016, at the Serra do Cipó, Santana do Riacho county, Minas Gerais state, Brazil (19° 28' S and 43° 58' W). This area is localized in the southern region of the Espinhaço mountain chain and it is characterized by rupestrian fields (Medina et al., 2007). The weather of the region is classified as CWB of Kóppen (1948), altitudinal tropical, with warm and rainy summers and a dry season that can last seven months (Ribeiro et al., 2009). A soil sample where the species were found was collected. Soil pH was measured in solution using a 1:2.5 soil/ water ratio (DM-22 Digimed pHmeter); sand, silt, and clay contents (dag kg-1) and organic material (dag kg-1) were determined using the pipette method. The region was characterized by acid and sandy soil, with low content of organic material (Table 1).
Leaf anatomy
Healthy, turgid, and fully expanded leaves from three individuals of each species, situated at minimum distance of 1 m among the plants were harvested. This guarantees that the material belongs to different individuals.
Fragments of 2 cm2 from the centre region of the leaves were fixed in 1 % (v/v) paraformaldehyde and 1 % (v/v) glutaraldehyde in 0.05 M sodium cacodylate buffer, at 9-11 am. The tissue was dehydrated through a graded series of ethanol and embedded in LR White resin (Voznesenskaya et al., 2013). The leaf anatomy was studied by transverse sections (2-3 um) obtained using a rotative microtome (model MRP-09 LUPETEC) and stained with 1 % toluidine blue.
A total of 36 observations per species were analyzed: three different sections and four images per sections for each specimen. The distance (um) and the number of cells between two veins, percentage of M and BS cells, and M:BS ratio were determined. The percentage of M and BS cells was calculated using a stereological grid of 500 random points layered on transverse section images and counting the proportion of points in M and BS cells in comparison to the total points (Mckown and Dengler, 2007).
RESULTS
The leaf transverse sections showed that the four Apochloa species had uniseriate epidermis with bulliform cells with rounded shape in the adaxial epidermis. Projection of the sclerenchyma connects the vascular bundles to the adaxial and abaxial epidermis. The mesophyll shows intercellular spaces and an arrangement of cells around the vascular bundles, with enlarged and uneven BS cells (Fig. 1).
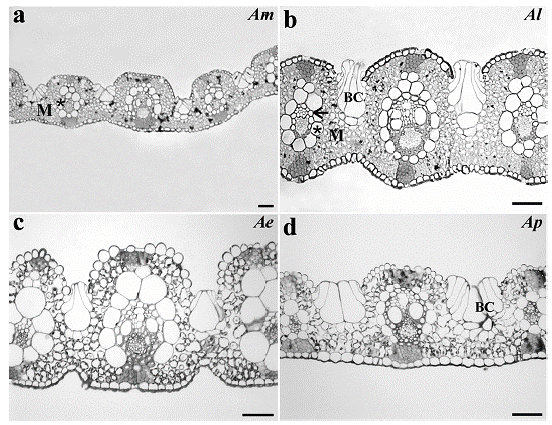
Figure 1 Leaf transverse sections from a. Apochloa molinioides (Am), b. Apochloa lorea (Al), c. Apochloa euprepes (Ae), and d. Apochloa poliophylla (Ap). M, mesophyll; asterisk, bundle sheath; BC, bulliform cell. Scale bar= 50 μm.
All the species analyzed presented two layers of cells around the vascular bundle, being the inner layer of thick-walled cells characterized as mestome sheath and the outer layer as parenchymatous bundle sheath. In spite of the enlargement of BS cells, only few chloroplasts organized in a centrifugal position were observed, while in the M cells the organelles showed a periphery position pattern (Fig. 2).
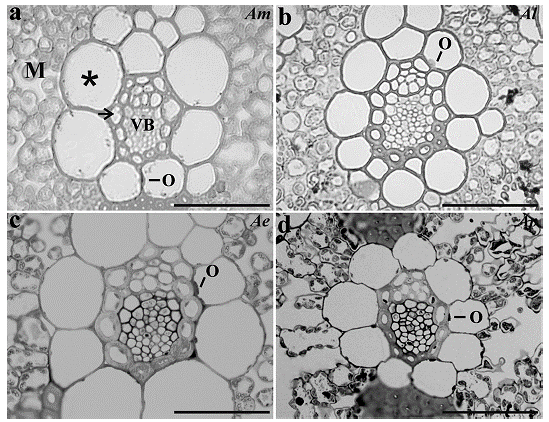
Figure 2 Bundle sheath cells organization from a. Apochloa molinioides (Am), b. Apochloa lorea (Al), c. Apochloa euprepes (Ae), and d. Apochloa poliophylla (Ap). M, mesophyll; asterisk, bundle sheath; arrow, mestome sheath; VB, vascular bundle; O, organelle. Scale bar= 50 μm.
Leaf anatomical traits showed significant difference between the species (Table 2). The highest distance between veins (519.0 μm) was observed for A. molinioides, while lower values were observed for A. euprepes and A. poliophylla (217.5 and 200.8 um, respectively). In relation to the number of M cells between two veins, the lower values were also recorded for A. poliophylla and A. euprepes. Although these species had the lowest values of distance and number of cells between veins, and percentage of mesophyll cells, A. euprepes showed the highest percentage of BS cells (24 %), which determined more than 30 % decrease in the M:BS ratio. The enlargement of BS at the expense of M cells is a characteristic strongly related to the establishment of C4 pathway.
Table 2 Leaf anatomical characteristics of Apochloa species from Serra do Cipó, Santana do Riacho county, Minas Gerais state, Brazil.
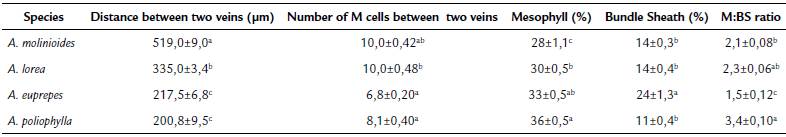
Values represent mean ± SE, n= 36 observations per species. In each column, values followed by the same letter are not different (one-way ANOVA and the Tukey's test; p<0.05).
DISCUSSION
In all Apochloa species analyzed an enlargement of the bundle sheath (BS) cells was found, an anatomical leaf trait commonly observed in intermediate species allowing to establish a C4 pathway (Gowik and Westhoff, 2011; Muhaidat et al., 2011). In grasses, BS cells (parenchymatous cells) are organized in one or two layers, so that in the species with two layers, the inner layer called mestome sheath has small cells with suberized thick walls and absence of chloroplasts or other organelles, and it is located between the vascular tissue and the outer layer of parenchymatous bundle sheath (O'Brien and Kuo, 1975; Hattersley et al., 1976; Dengler et al., 1985; Lundgren et al., 2014; Miyake, 2016), a typical organization observed in Apochloa species.
The emergence of Kranz anatomy is characterized by the presence of a concentric layer of cells around the vascular bundle, which allows the metabolic shuttle between M and BS cells, and in consequence the emergence of CO2 concentration mechanism (Sage et al., 2012; Lundgren et al., 2014). Thus, the presence of enlarged and radial arranged bundle sheath cells (parenchymatous cells) around the bundles can be considered a rustic Kranz anatomy (Muhaidat et al., 2011); with the exception that they are not surrounded by a concentric layer of mesophyll cells (Rawsthorne, 1992) and a few intercellular spaces. This concentric organization is common in C3 grasses related to C4 groups of the family Poaceae (Lundgren et al., 2014), as observed for Apochloa species. Besides that, the enlargement of the bundle sheath cells may represent an adaptation for C4 physiology, once this cell needs to accommodate more and larger chloroplasts to guarantee an efficient photosynthetic cycle (Lundgren et al., 2019).
During the development of C4 pathway, the reduction in the distance and in the number of cells between two veins and the decrease of the M:BS ratio are common, which enables the metabolite shuttle between mesophyll and bundle sheath cell compartments (Lundgren et al., 2014).
However, even with the reduction observed for the Apochloa species, our findings are still in the range of values found for C3 grasses (Christin et al., 2013). The C3 grasses Dicanthelium oligosanthes (Schult.) Gould and Panicum bisulcatum Thunb. had distance between veins of 206 and 240 um, respectively; figures more than 50 % higher than the studied C4 grasses (Khoshravesh etal., 2016). High distance between veins was also observed for the C3 grass Jansenella griffithiana (Mull. Hal.) Bor, with a mean of 471 um (Bianconi et al., 2020). In relation to the number M cells between veins, C3 PACMAD species usually have three times more cells than C4 PACMAD ones (Ermakova et al., 2020). This pattern was observed for the C3 and C4 grasses Neurachne alopecuroidea R. Br. and Neurachne minor S. T. Blake, with 6.5 and four cells, respectively (Khoshravesh et al., 2020).
In fact, previous studies already pointed out that the genus Apochloa does not present specialized chloroplasts in the bundle sheath cells (Zuloaga et al., 2010), as it was observed in all four species analyzed here. The ability to fix and reduce CO2 in the BS cells is another step of BS cell activation and participation in the photosynthesis process (Gowik and Westhoff, 2011; Sage et al., 2014). In order to confirm the presence of photosynthetic enzymes in cells, studies of ultrastructure and enzymatic immunolocalization are needed. However, scarce organelles, mainly chloroplasts, found in these parenchymatous cells may indicate that the engagement in carboxylation activity of photosynthesis is not the main function.
In this case, the enlarged BS cells may have another function, related to the environmental conditions in the area of collection. Sandy soils, poor in organic material, have a low capacity of water retention. Thus, the weather and soil characteristics provide low water availability for the most part of the year, even during the rainy season (Giulietti et al., 1987; Castro and Menezes, 1995). In fact, bundle sheath cells of C3 tropical grasses have functions related to water supply (Sage, 2001; Griffiths et al., 2013). It is related that C3 PACMAD grasses have a high tolerance to the increase of evaporative demand through the investment in bigger BS cells (Griffiths et al., 2013). Under high temperature or low air humidity, and low water availability bundle sheath cells may store and regulate the water flux to the mesophyll, maintaining the leaf hydraulic integrity (Griffiths et al., 2013), besides preventing air entering the xylem (Sage, 2001; Leegood, 2008). In this context, the water reserve function of BS cells has been considered a predisposition for C4 evolution in grasses, favouring the occupation of tropical and subtropical environments (Sage, 2001; Griffiths et al., 2013), center of expansion of C4 grasslands (Edwards et al., 2010; Osborne and Sack, 2012).
Apochloa species showed a rustic Kranz anatomy, with the presence of enlarged BS cells. Although they presented a typical C3 leaf anatomy and most of the organelles (chloroplasts) are restricted to M cells, the percentage of tissue related to BS cells may facilitate the establishment of C4 pathway in C4 genera related to these Apochloa species. Thus, more ultrastructural and phylogenetic investigations are needed in order to understand if those characteristics are coordinated with modifications of enzymes compartmentalization and the emergence of intermediate and C4 species in the clade related.
CONCLUSIONS
The presence of rustic Kranz anatomy in the Apochloa species analyzed could indicate facilitation to the development of the C4 photosynthesis in this group or may represent a trace of an ancestral C4 condition. However, ultrastructural and enzymatic analyses are required to confirm the photosynthetic metabolism of this species.