INTRODUCTION
Considered one of the biggest public health problems in the world, the leishmaniasis is caused by protozoa of the genus Leishmania Ross, 1903. One of the clinical spectra present in the Americas is the cutaneous form (ACL), endemic in Brazil (Savoia, 2015). In the South Region of Brazil, most human cases occurred in the state of Paraná, with 4582 reported cases between 2007 and 2018, according to data from the Ministry of Health of Brazil. One of the surroundings in which autochthonous transmission of ACL occurs in the state of Paraná corresponds to the Ribeira de Iguape River Valley, where for over a century the disease has been registered along with report of the species Leishmania (Viannia) braziliensis Vianna, 1911 that was isolated from patients (Castro et al., 2005).
Ribeira River Valley is a region heavily influenced by anthropogenic changes. The Phlebotominae sand fly populations have changed their profile in relation to previous research over recent decades, and Lutzomyia (Nyssomyia) intermedia (Lutz and Neiva, 1912) have been currently found in abundance and considered the vector of Leishmania in domestic, peridomestic and sylvatic environments (Forattini et al., 1976; Castro et al., 2005; Gonçalves et al., 2019).
Lutzomyia intermedia is widely distributed in Brazil, from the North and Northeast to the South. It is also present in northern Argentina, Paraguay and southern part of Bolivia (Andrade Filho et al., 2007; Salomón et al., 2016). Adopting morphological criteria, Lutzomyia (Nyssomyia) neivai (Pinto, 1926) was redescribed by Marcondes (1996)) as a different species of L. intermedia. The author adopted the relationship between genital and extragenital structures to distinguish males of L. intermedia and L. neivai, such as ejaculatory ducts in males and the spermathacae and cibarial teeth of females.
Besides morphometric data, Marcondes (1997) has also studied mitochondrial DNA sequences and observed two phylogenetic lineages of L. intermedia: L. intermedia s.s. and L. neivai. Studies evaluating cryptic speciation, and the level of genetic structuring of populations of Phlebotominae in endemic areas, are epidemiologically relevant since they show how these species or their lineages differ in significance as vectors of Leishmania (Azevedo et al., 2000; Bejarano et al., 2009; Freitas et al., 2018). Molecular data may contain enough variations to assist in these matters, revealing variations between populations from different geographic areas that are not distinguishable by morphology and those that constitute species complexes (Chan-Chable et al., 2019; Lozano-Sardaneta et al., 2020).
The RAPD technique can be used to determine the level of genetic variability in sand flies and clarify doubts regarding the current species complexes, which are difficult to distinguish solely by morphological features. This technique has been used to investigate the genetic characteristics of insect vectors including the taxonomic relationships between cryptic species (Adamson et al., 1993; Fraga et al., 2011), intra- and interpopulational variability (Meneses et al., 2005; Rocha et al., 2007; Seblova et al., 2013) and the identification of populations resistant or susceptible to insecticides (Hiragi et al., 2009) or phytochemicals (Sharma et al., 2017).
Considering the possibilities of Leishmania transmission in different ecotypes in the Ribeira Valley and considering the high-density of L. intermedia in these environments, we raised the hypothesis that the vector role in the region is influenced by the degree of genetic structure of the populations of this sand fly. Therefore, may L. intermedia show enough variation to maintain different parasite transmission cycles? If these variations exist, may support occurrence of gene flow between populations of different ecotypes, areas or lineages? And this aforementioned variation, may also support the differentiation between L. intermedia s.s. and L. neivai, or among populations of different ecotypes or areas?
In this study, using RAPD markers, we analyzed the genetic variability of L. intermedia in three environments (house, peridomicile and wild), in two areas of Ribeira River Valley in the state of Paraná: the municipalities of Cerro Azul (a new transmission area) and Adrianópolis (an endemic area), with 164 and 118 cases of ACL reported between 2007 and 2018, respectively. We seek to assess the relationship among the domestic, peridomestic and sylvatic parasite transmission cycles. These markers were used to assess the level of divergence between the populations that comprise the taxon L. intermedia: L. intermedia s.s. and L. neivai, that are present in the region.
Thus, this study contributes to a better understanding about genetic variations of sand flies, detected by the technique of RAPD, to disclose the possible roles of these vectors in the epidemiology of ACL in the Ribeira de Iguape River Valley.
MATERIALS AND METHODS
Sampling area and sand fly captures and identification
Sand flies were captured in Cerro Azul (24°49'08"S, 49°15'37"W and 324 m.a.s.l.) and Adrianópolis (24°39'42"S, 48°59'30"W and 181 m. a. s. I.). These municipalities belong to the state of Paraná, southern Brazil, in the Ribeira de Iguape River basin (Fig. 1). Originally formed by covers of dense ombrophilous and mixed ombrophilous forest, this region is currently occupied by secondary forest, planted forest and by agricultural and extractive activities, as well as by the growth of extractive and transformation industries (IPARDES, 2021). Another anthropogenic influence in the region is the passage of the Bolivia-Brazil gas pipeline (1998-2009).
Figure 1 Geographical localization of the sand fly captures: municipalities of Cerro Azul and Adrianópolis, State of Paraná, Brazil
For the capture of sand flies, CDC-type light traps (Sudia and Chamberlain, 1962) were installed in areas with the occurrence of ACL transmission, in different ecotypes: inside the house, in the peridomicile and in the wild (forest). The samples were collected during the months of December 2008 and February, May and November 2009, between 7 p.m. and 12 a.m. (midnight).
For identification, the last abdominal segments of sand flies were clarified in 20 % potassium hydroxide solution. After 24 h, the sand flies were observed through an optical microscope and identified according to characteristics following the key proposed by Young and Duncan (1994). Marcondes' (1996) criterion was adopted to distinguish between the two lineages of L. intermedia; females with more than 11 spermathecae rings were identified as belonging to L. intermedia s.s., while the remainder were identified as L. intermedia s.l., together with all the males.
Molecular/RAPD analysis
Samples were subjected to molecular analysis by RAPD-PCR (Polymerase chain reaction), selected from 52 individuals (45 males and five females of L. intermedia s.l. and two females of L. intermedia s.s.) out of a total of 403 previously collected specimens (Gonçalves et al., 2019).
The DNA of the samples was extracted as proposed by Loxdale and Lushai (1998). Briefly, samples were triturated in 300 μL of 5 % Chelex100 within sterilized 1.5 mL microtubes with plastic pistils. Then, they were stirred strongly (for 15 s) and spun (19 500 xg) for 20 s. After that, they were incubated in a water bath (80 °C) for 30 min and then shaken again and centrifuged (19 500 xg/20 s). The supernatant containing the DNA was transferred to the new tubes and stored at -20 °C.
Then, the DNA were amplified by RAPD-PCR, using the following primers: A10 (5'-GTGATCGCAG-3'), A2 (5'-TGCCGAGCTG-3'), A3 (5'-AGTCAGCCAC-3') and A9 (5'-GGGTAACGCC-3') (Tibayrenc et al., 1993). The reaction was performed in a final volume of 25 μL containing 1.5 μL of DNA (1.64 ηg), 16.8 μL of ultra-pure sterilized water, 2.5 uL of buffer (10X), 2.5 /μL of primer (5 /μM), 0.75 μL of MgCl2 (50 mM), 0.5 /μL of dNTP (10 mM) and 0.45 /μL of Taq DNA-polymerase (Invitrogen, Carlsbad, CA, USA) (5 U/μL).
Amplification was performed using a MJ Research® thermocycler (Watertown, MA, USA), with an initial denaturation at 94 °C for five min, followed by amplification with 45 cycles of denaturation (94 °C, one minute), annealing (36 °C, one minute) and polymerization (72 °C, two minutes), and a final extension at 72 °C for seven minutes. After amplifying the fragments by RAPD-PCR, the products obtained were submitted to separation according to molecular weight by horizontal electrophoresis on 1.5 % agarose gel in 1X TBE buffer (Tris-base, boric acid and EDTA pH 8.3) at 60 Volts for three hours. For size standards, we used the one kb and 100 bp Plus DNA Ladder Gibco markers.
The gels were stained with ethidium bromide (0.5 mg/ mL) and visualized under ultraviolet light (TFX-35M Gibco BRL UV Transilluminator). In addition, we photographed and then analyzed the gels.
Statistical/Data analysis
The amplification profiles were analyzed in groups of ten samples of the same type of ecotype for each marker (A10 and A2). Thus, for the phenetic analysis, representative samples of each observed polymorphism were chosen. Based on the patterns obtained following RAPD, the Jaccard distance (D)) was used to create data matrices, considering the presence or absence of bands (one or zero, respectively) (Jaccard, 1908). Each band of the RAPD gel was encoded with a number starting with one for the slowest band (which contained a larger DNA fragment); thus, each genotype is represented by a set of numbers for each primer. This coefficient is represented by D = 1 - [C/(2N - C)], where C is the number of common bands between genotypes i and), and N is the total number of bands in both genotypes. The units were grouped by the UPGMA (Unweighted pair-group method with arithmetical average) method, which is a hierarchical clustering model that allows the construction of dendrograms (Sneath and Sokal, 1973). The Mantel test of correlation of matrices (Mantel, 1967) was performed to observe the significance of the correlation between the genetic similarity matrix and the cophenetic matrix obtained from the data presented by the dendrograms. PCO was performed to test for possible clustering in the genetic similarity matrix. The dendrograms, Mantel's test and PCO were obtained using the program NTSYS pc 2.1 (Rohlf, 2000). The strength of the nodes in the dendrogram was evaluated by bootstrap analysis using the Bood 3.03 program (Coelho, 2005) with 10 000 permutations.
RESULTS
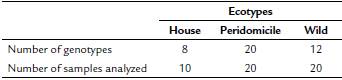
Of the four primers selected for this study, two (A10 and A2) generated genetic profiles that allowed us to identify the level of polymorphism in populations of L. intermedia s.l. In this study, the amplification profiles obtained using primers A3 and A9 have shown none of the polymorphism visualized in products amplified with the use of primers A10 and A2. Therefore, the samples of sand fly DNA amplified with these primers have not been submitted to phenetic analysis. However, the use of primers A3 and A9 generated characteristic fragments with molecular weights between 400 and 500 bp. Overall, 40 genotypes were identified within the population of the lineage L. intermedia s.l. studied in 50 sand flies, collected from three ecotypes in two municipalities (Table 1). The peridomestic ecotype presented the highest genotypic diversity.
Table 1 Total number of genotypes obtained by RAPD of Lutzomyia intermedia from the three different ecotypes in the municipalities of Cerro Azul and Adrianópolis.
The five L. intermedia s.l. females that were subjected to the RAPD technique, did not present relationship determined between the genotypes and the number of rings in the spermatheca of the females. Two females captured from the wild area in Cerro Azul had the same genotype, but one female had eight rings in the spermatheca, while the other presented nine rings. By contrast, three other females, also from the wild area of the same municipality, presented ten, nine, and seven rings respectively, in each spermatheca, and each showed different genetic profiles. In this way, the primers used in this research did not generate genotypes that maintained a relationship with morphological markers in L. intermedia s.l.
The genetic diversity of the population of L. intermedia s.l. studied was revealed not only by the number of genotypes identified, but also by analyzing the dendrogram generated by the matrix constructed from the profiles of DNA bands amplified with the A10 primer. The Mantel test showed r values (correlation between matrices) > 0.7 and p (significance test of r) < 0.01.
The dendrogram showed different degrees of similarity (30 to 80 %), according to the Jaccard coefficient for the same ecotype (Fig. 2). This suggests intraspecific polymorphism and the possibility of genetic flow among the studied populations.
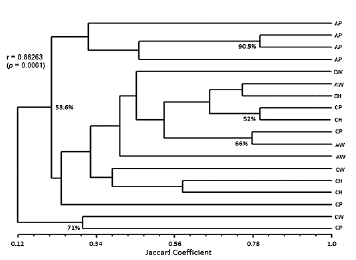
Figure 2 Dendrogram construction from data obtained by electrophoresis on 1.5% agarose gel, performed with primer A10 RAPD-PCR products. The specimens of Lutzomyia intermedia were captured in the municipalities of Cerro Azul and Adrianópolis. The dendrogram was generated by the program NTSYS-pc 2.1 with clustering by the UPGMA method and the Jaccard coefficient distance. Legend: house environment (CH), peridomicile (CP) and wild area (CW) of Cerro Azul and peridomicile (AP) and wild area (AW) of Adrianópolis. Bootstrap values (%) of each group are shown.
The PCO from the RAPD with the primer A2 clustered the sand flies in relation to their environment and municipality of collection (Fig. 3). Analyzing the graph, the samples from Cerro Azul were grouped together, and the samples from Adrianópolis formed another grouping. One specimen female classed as L. intermedias.s. lineage, collected in the wild in Adrianópolis, was grouped with the other sand flies from this municipality (L. intermedia s.l.).
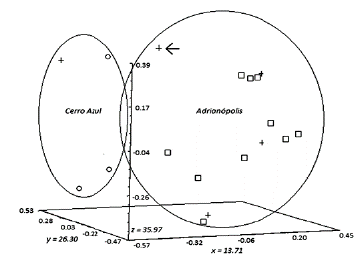
Figure 3 Principal Coordinate Analysis (PCO) of specimens of Lutzomyia intermedia from Cerro Azul and Adrianópolis. The graph was generated by the program NTSYS pc 2.1, from electrophoresis data performed with primer A2 RAPD-PCR products. Symbols: (°) house, (□) peridomicile, (+) wild. Specimen indicated by the arrow belongs to the Lutzomyia intermedia s.s. lineage.
DISCUSSION
Molecular markers, such as RAPD, have been used in several studies of sand flies in the New and Old World, including taxonomic identification and population genetics (Golczer and Arrivillaga, 2015).
Mukhopadhyay et al. (2000) have used RAPD to find species-specific DNA profiles of closely related and morphologically similar sand flies, Phlebotomus (Phlebotomus) papatasi (Scopoli, 1786) and Phlebotomus (Phlebotomus) duboscqi Neveu-Lemaire, 1906. One of the tested primers has generated amplifications with differential profiles between the two species, with a characteristic band of 700 bp present in P. papatasi, and one of 490 bp present in P. duboscqi. Regarding the present study, the fragments generated by primers A3 and A9 may constitute a species-specific, genus-specific or even ecotype-specific marker. Likewise, Adamson et al. (1993) have also identified a 320 bp characteristic band present in Lutzomyia youngi Feliciangeli and Murillo, 1987, which has allowed to differentiate from the nearby species, Lutzomyia spinicrassa Morales, Osorno-Mesa, Osorno and Hoyos, 1969.
In our study, RAPD markers did not generate genetic profiles that allowed us to identify the sand flies according to the morphological criteria used to distinguish the females of the taxon L. intermedia proposed by Marcondes (1996). This is contrasting to the study by Silva et al. (2011), who analyzed populations of Lutozmyia (Lutzomyia) longipalpis (Lutz and Neiva, 1912) in the state of Maranhão, northeastern Brazil, using RAPD, and divided the population according to the morphological markers found in males that they used to distinguish the existing varieties within this species. In the case of L. longipalpis, the possible existence of a species complex based on the morphological markers present in males has been justified by several genetic and behavioral studies (Souza et al., 2004b; Lima Costa et al., 2015; Vigoder et al., 2015; Souza et al., 2017).
The results obtained in this study, using primer A10, denoted the existence of gene flow within the study population (old and new transmission areas of ACL). Specimens collected in different ecotypes and different municipalities were grouped in the same phenetic subgroup with varying degrees of similarity, as the results obtained by similar studies involving Anopheles Meigen, 1818 species (Posso et al., 2003; González et al., 2007). The evidence of gene flow is denoted not only by analysis of the dendrogram, but also considering the observation that sand flies from different municipalities showed the same genetic profile. In this study, the occurrence of gene flow can be explained by the lack of substantial physical barriers between the two selected areas that are about 30 km distant.
Analogous studies have reported similar trends, e.g., the work proposed by Pinedo-Cancino et al. (2006). The authors used RAPD to verify microgeographic genetic variation in nine geographically separated populations (2060 km) of Anopheles (Nyssorhynchus) darlingi Root, 1926 in the municipality of Iquitos (Peru). The average genetic distance obtained demonstrated the genetic homogeneity between these populations and, thus, the presence of a high level of gene flow. As a counterpoint, the genetic homogeneity verified in populations of L. longipalpis in the northeast region of Brazil, is justified by Balbino et al. (2006) as the result of geographic isolation and restricted gene flow. This is compatible with the hypothesis that there is a single species of the sand fly in this area.
The municipalities ofCerro Azul and Adrianópolis belong to the same ecosystem, the Ribeira River Valley, and they border each other. The absence of geographic isolation of these populations allows sand flies to migrate and exchange genetic material among themselves in different areas. Furthermore, this process may have been facilitated by the impact of the construction of the Bolivia-Brazil gas pipeline, which runs through the municipality of Cerro Azul. In fact, large-scale anthropogenic interventions can influence the levels ofgenetic structuring in vectors populations and, as a consequence, have an impact on the transmission potential of pathogens (Burkett-Cadena and Vittor, 2018; Suesdek, 2019).
Considering the lack of barriers to flight, L. intermedia can move, specially helped by the environmental changes of the gas pipeline construction and might ensure the gene flow in the Ribeira Valley, Paraná. This hypothesis is supported by the dispersal potential that these insects possess. The L. intermedia dispersion and survival were studied by Galati et al. (2009) in Iporanga, endemic area of ACL in the Ribeira Valley of the state of São Paulo. They reported that the dispersal distances were 109 m for L. intermedia s.s. and 100 m for L. neivai. The maximum dispersal distance was 180 m for L. intermedia s.s., while they recovered L. neivai in a pasture 250 m away and another in a pigsty 520 m away, showing a trend to disperse to more open areas.
The RAPD analysis can also be a useful tool to distinguish insects depending on their breeding sites/locations. This is demonstrated by the graph generated by PCO using data generated by the A2 primer. It reveals two major subgroups: the first formed by sand fly samples from Adrianópolis, and the second consisting of samples captured in Cerro Azul. A study proposed by Dvorak et al. (2006) sought to clarify the taxonomic nature of Phlebotomus (Paraphlebotomus) sergenti Parrot, 1917 by developing colonies with populations of this sand fly from Turkey and Israel. They performed RAPD with the offspring, which indicated the formation of distinct subgroups related to the origin of each colony. However, there was a high level of variability within each subgroup.
In this study, the group that involved sand flies from Adrianópolis included samples from the forest together with peridomestic areas. In the Cerro Azul group, a sand fly from the forest belonged to the same group as three other samples from the inside of the house. These arrangements of specimens from ecotypes that differ from those in which they were prevalent can also be justified by the existence of migration and gene flow. After evaluating the genetic variability of four populations of Lutzomyia (Nyssomyia) whitmani (Antunes and Coutinho, 1939) from different areas of ACL transmission in Brazil using RAPD, Souza et al. (2004a) reported two main spatial groupings with a high level of intra-population variability: Corte de Pedra, Ilhéus (Bahia) and Serra de Baturité (Ceará) in the first group, and Martinho Campos (Minas Gerais) in the second. The vast majority of individuals of these populations were grouped according to their region of origin. However, unlike females, 13 % of males from Martinho Campos moved to Serra de Baturité, instead of Ilhéus. The non-uniformity in the genetic lineage of Martinho Campos is justified by the existence of sympatric populations in these regions, because individuals with intermediate profiles can be explained by the gene flow among these populations.
Here, the peridomicile was the ecotype with the highest genotypic diversity. This diversity can be explained by geographical conditions present at the collection sites. Meneses et al. (2005) compared three populations of L. intermedia from the same endemic area of ACL in the state of Rio de Janeiro, Brazil. Using RAPD, they were able to distinguish between two major subgroups, one related to the house or peridomicile and one related to the wild (woods). The latter was the most polymorphic, with the largest number of genotypes and a low degree of similarity. According to the authors, due to the high level of gene flow between different habitats, five individuals from the wild were grouped with the subgroup formed by sand flies from the house and peridomicile, and one individual from the house area was grouped with the subgroup of samples collected in the wild.
To explain the genetic diversity within and between populations of L. intermedia from the municipalities of Afonso Cláudio and Viana (state of Espirito Santo, Brazil), Rocha et al. (2007) mention the years of human colonization of the two sites (200 and 80 years, respectively), which may reflect the differences in the behavior of these populations. The authors point out that although there was a trend to group individuals collected in the same ecotype into the same phenetic group, this fact does not necessarily indicate a genetic separation between the populations. Moreover, according to Rocha et al. (2007), the high level of genetic organization of populations of L. intermedia in different ecotypes of the Viana may reflect the independence between the domestic and peridomestic transmission cycles of Leishmania, such that the genetic divergence of L. intermedia may be occurring because of changes in their original forest habitat due to the ability to adapt to a new environment.
The Ribeira Valley is a region heavily influenced by anthropogenic changes, with varying degrees of degradation in the original landscape. L. intermedia is a species that has great adaptability in modified environments, showing synanthropic behavior and attraction to domestic animals. These aspects favor the transmission of the parasite in the house and peridomestic area (Gomes et al., 1986; Saraiva et al., 2012).
The high genetic diversity verified herein cannot suggest an independence between the cycles of wild, peridomicile, and household transmission of ACL, because these ecotypes are very close to each other and lack the geographical barriers that could separate the populations according to ecotypes or strains (Saraiva et al., 2012; Gonçalves et al., 2019). Therefore, the high genetic variability shown for L. intermedia, combined with its high prevalence, supports L. intermedia as a vector of L. braziliensis in the Ribeira Valley.
Since it has shown to be the most prevalent sand ly species in the Ribeira River Valley region and has been considered one of the primary vectors of L. braziliensis in South America, L. intermedia has become an undisputed target for further studies.
The present investigation has met a part of these demands, by contributing to the genetic characterization of this species. Further investigation is important not only for new knowledge about its taxonomy and genetic diversity, but for further clarification on the participation in the Leishmania transmission cycle. Thus, underlying research involving molecular biology using techniques and resembling this study becomes compelling. Among these, we can mention the characterization of ribosomal DNA sequences (Almazán et al., 2020); that have used other markers recognized as useful in specific differentiation, such as the period gene (Freitas et al., 2018); and protein analyzes, such as those performed by mass spectrometry (Mathis et al., 2015).
CONCLUSIONS
In this study, a high genotypic diversity and a detectable gene low have been found among populations of L. intermedia that have been collected in the Ribeira River Valley Region, southern Brazil, through the application of RAPD. It was verified, in the studied area, that the highest level of L. intermedia polymorphism was found among the insects captured in the peridomicile. Furthermore, the data suggest that the occurrence of sand ly migrations among this ecotype, the wild and the house, allow the transmission of L. braziliensis between animals and humans in the Ribeira River Valley in both ecotypes, as well as along the investigated municipalities. This condition of vector species is corroborated by the high genetic variability found.
RAPD analysis has been frequently used to determine the level of genetic variability in various insect species. In public health, in addition to population genetics, the RAPD and other molecular biology techniques offer special interest regarding pathogen vectors, both to elucidate whether there is a relationship between the vector genotype and its vector competence, as well as to rule out doubts about the species complexes that present difficulties for identification by morphological characters. In this study, it has not been possible to obtain profiles that were related to morphological markers used to distinguish between the lineages that set L. intermedia. However, the analysis with different markers has enabled to group populations according to their bio-geographical origin.















