Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería e Investigación
versão impressa ISSN 0120-5609
Ing. Investig. v.30 n.2 Bogotá maio/ago. 2010
Carlos Alberto Guerrero Fajardo1, Iván David Osorio León2 and Fabio Emiro Sierra Vargas3.
1 Chemical. Mechanical Engineer. M.Sc., in Environmental Engineering. Ph.D. Sc., in Chemical Engineering, School of Science, Universidad Nacional de Colombia. caguerrerofa@unal.edu.co 2 Student Chemical Engineering, School of engineering, Universidad Nacional de Colombia. idosoriole@unal.edu.co 3 Mechanical Engineer. Ph.D. Sc.,in Engineering - Renewable Energy, School of ngineering, Universidad Nacional de Colombia. fesierrav@unal.edu.co
ABSTRACT
Problems arising between biofuels and food as raw materials have led to investigating the use of inedible raw materials for their production. This work was aimed at studying the effect of temperature on converting castor oil in biodiesel production. Oil transesterification with methanol was carried out using an alkaline catalyst (0.5% NaOH - water solution) for 1 hour using a 6:1 alcohol/oil molar ratio, at atmospheric pressure and taking temperature as a free variable. The temperature was evaluated at 68°F, 86°F, 104°F and 122°F. The reaction products were analysed by gas chromatography (CG-FID) for quantifying the fatty acid methyl esters (FAME) present. The results showed different dispersion depending on temperature, finding that 122°F resulted in less dispersion than the others. CG-FID analysis showed that most FAME content was reached at 122°F, such temperature giving the highest ricinoleic acid conversion rate. Gas chromatography also revealed that reaction time was adequate, in process conditions, for obtaining ricinoleic acid-based 94.26% conversion.
Keywords: castor oil, biodiesel, transesterification, alkaline catalysis.
Received: may 27th 2009
Accepted: jun 20th 2010
Introduction
The partial substitution of petrodiesel, or petroleum-derived diesel, for renewable energy sources is a worldwide trend and such replacement entails positive environmental effects including the reduction of pollutants in cities and reducing greenhouse gases(CORPODIB, 2003)
Biofuels are amongst the alternatives that further development and attention have brought, including biodiesel as a very promising solution for liquid fuels (Conceicao, 2005). Biodiesel fuel involves has benefits, such as working in any conventional diesel engine without modification, can be used pure or mixed with diesel, contains no sulphur, reduces CO emissions and has no net CO2. emissions but may improve engine life (CORPODIB, 2007; Guerrero Rodríguez, 2003).
However, one of the major social problems involving biodiesel has been food security threat, as some of the raw materials used in the process are also an important part of the food market (Guerrero Rodríguez, 2003; Demirbas, 2008).
Using non-food raw materials for biofuel production has been gaining greater importance in response to this problem, specifically biodiesel (Guerrero Rodríguez, 2003). Castor plant seeds have thus emerged as a very attractive solution as they contain about 40% weight (wt) in castor oil which is characterised by containing about 90% wt ricinoleic acid, thereby making this oil the one having the highest proportion of fatty acids of all vegetable oils (Conceicao,2005).
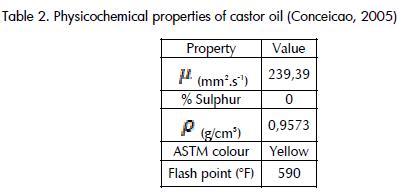
Transesterification of fatty acids contained in castor oil is a chemical process reducing the viscosity of the oil through the formation of methyl esters from fatty acids (as fatty acids react with methanol)and better flow properties for use as fuel in engines (Demirbas,2002), especially by reducing viscosity from 239.39 to 13.75 mm2.s-1, according to Conceicao (2005). Among the variables to be controlled during fatty acids' transesterification reaction it has been found that the water content of oil and free fatty acids have an impact on the formation of soaps and catalyst consumption,thereby reducing its effectiveness (Isayama, 2003, Demirbas, 2007). Some other variables discussed in the literature which influence the development of free fatty acid transesterification are the type of catalyst (Demirbas, 2007, Demirbas, 2005), alcohol (Demirbas, 2008) and molar ratio of oil to alcohol (Guerrero Rodríguez,2003).
As with any process having an impact on the economy and societ it is very important to find ways of optimising it so that its implementation can become economically feasible.
This work contains a description of the experimental procedure carried out to transform castor oil into biodiesel at different temperatures.Reaction conditions different to temperature were thus based on previous work and temperature was changed to four differrent values. This procedure led to identifying the effect of this variable on reaction performance. The temperature allowing the greatest biodiesel production was then identified based on chromatographic analysis of reaction products.
Experimental work
The experimental work was carried out in the Chemistry Department's Biomass and Energy laboratories at the Universidad Nacional de Colombia in Bogotá.
Castor oil (density: 0.957 g/ml) and 99.5% methanol (0.7918 g/ml) were taken as raw materials for the study and 0.5% commercial grade NaOH by weight was used as catalyst. According to Martinez Avila (2007), it has been found that 6:1 is the alcohol/oil molar ratio most improving reaction performance. The oil's composition and other properties of interest are presented below.
The transesterification reaction was performed in a 500 ml glas batch reactor equipped with a water-cooled vertical reflux condenser and a glass jacket which let water flow from a thermostat, thereby controlling the temperature inside the reactor (see Figure 1).

A conduit to the environment located at the top of the reflux condenser ensured that the pressure within the system was the same as Bogota's barometric pressure (560 mmHg). It also had a heating plate (on which the already-described reactor rested) with magnetic stirrer to provide agitation and ensure the required mixture for initiating the reaction.
The reaction mixture was not intended to occupy the reactor's entire volume, anticipating unwanted difficulties in the course of the reaction and as a security measure; certain amounts for occupying approximately 40% (200 ml) of the available volume were thus used:
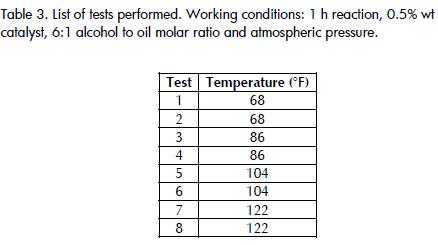
The reaction was carried out for one hour at four different temperatures to determine the effect of temperature, namely at68°F, 86°F, 104°F and 122°F. A replica was also made for each temperature. After an hour, the reaction was neutralised with 1 ml HCl (37% wt/wt), this being the quantity needed to stop th reaction. This amount was determined by taking into account the amount of NaOH which acted as a catalyst and which would neutralise free fatty acids, determined by means of acid value measured according to American Society for Testing and Materials (ASTM D1980-87, 1998).
The products obtained in the reaction were led to a decantation funnel and left there for a period of about 12 hours, after which there was separation into two phases where the higher percentage phase (light phase) was the biodiesel phase and the other was glycerine (heavy phase).
The glycerine present in the funnel was separated from the biodiesel phase and the latter was washed (consisting of liquid-liquid) with 10% wt/wt CH3COOH aqueous solution as washing agent at 68°F, applied in a 2:1 volumetric ratio regarding biodiesel. This was subjected to three washes with gentle stirring for 5 minutes, according to Martinez Avila (2007).
After washing, the biodiesel was characterised by measuring physical constants such as density and refractive index (Table 4).
Gas chromatography (GC-FID) characterisation was used for identifying the degree of reaction conversion as a function of FAME presence in such products (Guerrero, 2009).
Results
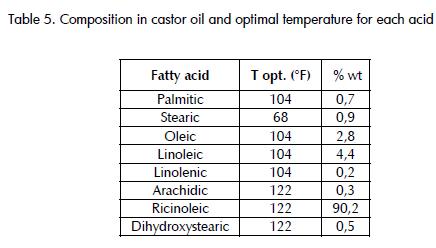
Table 5, shows the results obtained for each test, representing the percentage content of FAME for each acid in the biodiesel samples.
A chromatogram of castor oil in its initial state is presented which allows observing that methyl ester content was practically zero inside the reactor at the beginning of the reaction (Figure 2). The chromatograms for two biodiesel samples obtained at 86 and 122°F are also presented (Figures 3 and 4 respectively) showing the chromatography peaks for converting fatty acids to methyl esters.
Analysis and Discussion
The high percentage of ricinoleic acid methyl ester (84.58% on average) in all samples analysed here is worth noting as is the FAME for linoleic acid (5.85% on average) and FAME for oleic acid(5.02% on average). No significant difference in FAME production with temperature was seen. An assessment was thus made on the difference between the dispersion of results from one temperature to another to improve correlation and establish more precisely the effect of temperature on FAME production. Figures 5 to 12 show that the highest temperature dispersions were obtained at 68°F and 104°F and the remaining temperatures (86°F and 122°F) had the lowest dispersion. It is worth mentioning this data pattern since dispersion is a synonym for the reliability of results, determination precision being better as the dispersion of the results becomes lower. According to the above, it can be seen that the results for tests at 86°F and 122°F were those providing greater accuracy and and therefore reliability, the latter temperature having the least dispersion between tests. However, pairs of values were averaged for each temperature to make comparisons between tests and observe trends in behaviour.
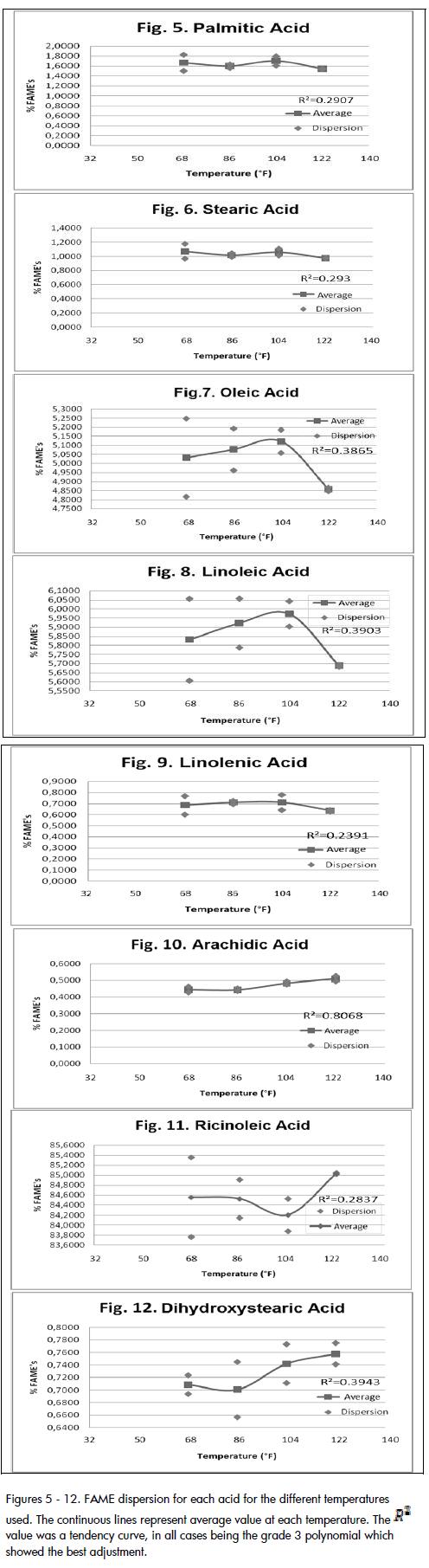
Analysing these graphs revealed several interesting points. It was seen that the highest FAME content for each acid was different for each temperature used. For example, there was increased FAME presence in samples subjected to 104°F for palmitic, oleic, linoleic and linolenic acids (named group 1); however, highest FAME content was achieved in samples at 122°F for arachidic, ricinoleic and dihidroxiesteáric acid (group 2). This observation suggested two possible options for a thermal reaction to be carried out,[4] namely working at 104°F favoured fatty acid transesterification group 1 or working at 122°F favoured the formation of methylesters in acids from group 2.
This dilemma was solved by comparing the presence of group 1 and 2 acids in castor oil, as group 2 included the most important acid[5] having most weight in the reaction and this group shoul have been favoured. A gain in conversion of the acids from this group (primarily ricinoleic) meant an overall gain for the reaction aving greatest importance than if it had been for group 1.
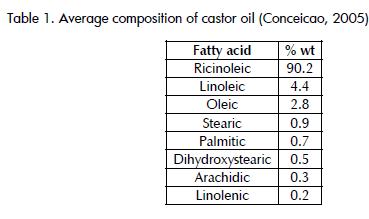
The information in Table 1 allowed weighting to indicate which group of fatty acids had most weight in castor oil and so define what the most suitable reaction temperature should have been.
Adding the compositions by weight of acids belonging to group 1 found that these acids represented 8.1% of the oil while group 2 accounted for 91.0 %, showing that the reaction temperature most positively affecting conversion was 122°F.
The good level of progress presented by the reaction for all temperatures should be noted, especially for 122°F, this being the temperature at which ricinoleic acid most reacted (the acid having increased presence in castor oil) to become transformed into methyl ricinoleate (Table 4 , showing FAME content formed from this acid). It should be noted that the percentage of FAME for each acid was close to the corresponding composition in Table 1 for different temperatures. This meant that virtually all the acids reacted with methanol to produce biodiesel and also that a 1 hour reaction time was enough time for the reaction to be carried out in the conditions used here. For example, the first test for ricinoleic acid at 122°F, shown in Table 4, shows 85.02% FAME and, according to Table 1 , its share in the oil is 90.2%. According to the above, 94.26% of the ricinoleic acid reacted at the established reaction conditions and at 122°F, this being a percentage showing an acceptable degree of reaction progress.
Conclusions
The work led to concluding that the data obtained for the various tests showed different degrees of dispersion, the most precise tests having been conducted at 122°F (lower dispersion) followed tests at 86°F.
It was also concluded that 122°F was the most suitable temperature for the transesterification reaction in the reaction conditions used here because maximum conversion to FAME of the acid having increased presence in castor oil (ricinoleic acid) happened at this temperature.
A reaction time of 1 hour was enough to obtain conversion of almost all fatty acids initially present in the oil.
Acknowledgments
The authors would particularly like to mention to Antek SA chemical laboratory for its assistance with the chromatographic analysis of biodiesel samples.
Alizadeh, A. M., Reza, M. P., Shanrokhi, M., Analysis of control structure for recycled reaction/separation processes with first order reaction., Petroleum and Coal, Vol. 48, 2006, pp. 48-60. [ Links ]
American Society for Testing and Materials., ASTM D1980-87. Standard Test Method for Acid Value of Fatty Acids and Polymerized Fatty Acids. [on-line], 1998. www.astm.org. [ Links ]
Conceicao, M., Candeiab, R., Silvac, F., Bezerrab, A., Fernandes, V., Souzab, A., Thermoanalytical characterization of castor oil biodiesel., Renewable & sustainable energy reviews, Vol. 11, No. 5, June, 2007, pp. 964-975. [ Links ]
CORPODIB y INDUPALMA S.A., Programa estratégico para la producción de biodiesel -combustible automotriz- a partir de aceites vegetales., Bogotá: Unidad de Planeación Minero energética, 2003. [ Links ]
CORPODIB., Estado del Arte de las Tecnologías de Producción de Biodiesel., Bogotá: s.n., 2007, pp. 323-326. [ Links ]
Demirbas, A., Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey., Energy Conversion & Managment, Trabzon, Turkey: s.n., 2002. [ Links ]
Demirbas, A., Biodiesel production from vegetable oils via catalytic and non.catalytic supercritical methanol transesterification methods., Progress in Energy and Combustion Science, Elsevier, 2005. [ Links ]
Demirbas, A., Comparison of transesterification methods for production of biodiesel from vegetable oils and fats., Energy Conversion & Managment, Trabzon, Turkey: s.n., 2007. [ Links ]
Demirbas, A., Biofuels sources, biofuel policy, biofuel economy and global biofuel projections., Energy Conversion & Managment. Trabzon, Turkey: s.n., 2008. [ Links ]
Demirbas, A., Production of biodiesel fuels from linseed oil using methanol and ethanol in non-catalytic SCF condition. Trabzan: Elsevier, 2008, Biomass & Bioenergy. [ Links ]
Guerrero, C. A., Sierra, F., Olmos, G., Aceite de Higuerilla alternativa como combustible., AIMUN (ed.), 1st Ed, Bogotá-Colombia, Universidad Nacional de Colombia, 2009, pp. 49-70. [ Links ]
Guerrero, A. M., Narváez, P.C., Prospectiva de la producción en Colombia de oleoquímicos derivados del aceite de Higuerilla., Bogotá: BSc thesis, Universidad Nacional de Colombia, 2003. [ Links ]
Isayama, Y., Saka, S., Effects of Water on biodiesel fuel production by supercritical methanol treatment., Bioresource Technology, Japon, Elsevier, 2003. [ Links ]
Martinez O., Sánchez F. J., Suárez, O. Y., Producción de ésteres etílicos a partir de aceite de palma RBD., Revista de Ingeniería e Investigación, 2007. [ Links ]











 texto em
texto em 


