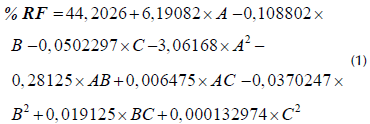Introduction
Today, there is a high dependence on oil for the production of fuels. This industry includes numerous processes, such as exploration, drilling, extraction, refining, etc., all of which require a high consumption of resources (Sayaddi et al., 2022). Fuels obtained from petroleum are an environmental concern, given that combustion results in high amounts of greenhouse gases and acid rain (Nandhini et al., 2022). Since the beginning of the 21st century, the growing energy demand has sparked great interest in the production and use of biofuels (Bosu et al., 2022). One of the biofuels that has impacted the current economy is biodiesel, a renewable energy source. It is estimated that the annual global production of biodiesel is approximately 35 billion liters (Naylor and Higgins, 2017). These fuels are composed of mixtures of methyl esters derived from the fatty acids that are part of the triacylglycerides from both vegetable oils and animal fats (Verma and Sharma, 2016), which have physicochemical properties similar to those of commercial diesel (Alptekin et al., 2012).
Belén (Nariño, Colombia) is a township with more than 2 500 inhabitants, which is located at 1°35'43" N, 77°00'57" W. Its economy depends on the production of leather, as more than 85% of its inhabitants participate in this activity. Currently, there are 43 tanneries in the urban area, and an average of 29 000 cowhides is treated every month. In this township, the leather industry has grown by tradition. These activities have a high environmental impact due to the amount of waste produced, including the tallow obtained in the fleshing stage of the leather tanning process, which accounts for 60 ton/month of the total residues, which are disposed of in dumps in the open or on the banks of the rivers (Díaz-Burgos, 2019). These substances contaminate water sources because they are very stable and change the composition of the water through oxidation (Vidales-Olivo et al., 2010). It is therefore necessary to develop technologies in order to utilize these resources. The fats present in tallow also contain between 75 and 80% of triacylglycerides, which makes them suitable for the production of biodiesel for vehicular applications with internal combustion engines (Sánek et al., 2015).
Biodiesel is formed by transesterification reactions, in which the triacylglycerides present in vegetable oils and animal fats react in the presence of KOH with an alcohol, thus obtaining alkyl esters as by-product (Ranjitha et al., 2020), which correspond to each of the fatty acids that form the triacylglycerides and glycerin (Knothe and Razon, 2017). Research has also been conducted on the use of enzymatic methods for the production of biodiesel with animal fat (Pollardo, 2017). Several sources of raw materials are used, the most common being: oil seeds such as soybean, rapeseed, palm oil, and bovine tallow from the food industry (Yuan, et al, 2017). Therefore, the objective of this work was the obtaining of biodiesel from bovine tallow, a solid waste generated in the leather tanning process.
Materials and methods
Reagents and materials
Hexane 95% alkane mix, ammonium sulfate (99,5%), potassium hydroxide (85%), methanol ACS (99,9%), sodium sulfate (99%), phenolphthalein solution (1%), acetic acid (99,5%), and chloroform ACS (98%) were obtained from Panreac. Ethanol (99,8%), potassium iodide (98%), and potassium iodate (99,5%) were obtained from Merck. Anhydrous sodium carbonate was obtained from Carlo Erba. Isopropanol (99, 5%) was obtained from Aldrich.
The materials used were a homemade pressure extractor, a magnetic stirrer hot plate (Heidolph Instruments 101142642) and a Radwag analytical balance (AS 220/C/2 366202).
Methods
Samples: Tallow was collected at the end of the fleshing stage from La Sociedad Curtimebres de Cueros Belén [The Belén Leather Tanning Society], in the town of Belén, Nariño, Colombia. 1,0 kg samples were collected in plastic bags and then frozen in order to delay the decomposition process until the moment of processing (Ranjitha et al., 2020). The solid wastes were washed with distilled water and solid ammonium sulfate (99,5%) for approximately 30 minutes. Acetic acid (99,5%) was then added until a pH value close to 7 was observed. The residues were cut manually into pieces measuring approximately 1 cm, which were then packed and stored in a refrigerator at -4 °C.
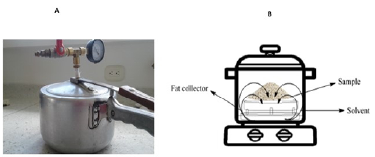
Fat extraction with pressurized solvent: An extraction technique involving a pressurized solvent (Figure 1a) was implemented for the fat extraction procedure. A sample holder was incorporated to this effect, as well as a container used to collect the extracted fat. The solvent was deposited at the bottom, outside of the grease collection container (Figure 1b).
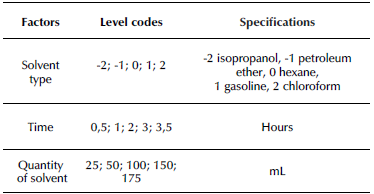
To optimize the fat extraction process, a central composite 22 design with two star points was used. The factors were the type of solvent (A), the extraction time (B), and the amount of solvent (C). The response variable was the percentage of fat recovery, as shown in Table 1, where the levels for each one of the factors, as well as their corresponding) units have been included.
According to the experimental design, between 25 and 175 mL of isopropanol (-2) solvent, petroleum ether (-1), hexane (0), gasoline (1), and chloroform (2) were added to the pressure system. Later, 100 g of tallow were added to the sample holder, and the container was hermetically sealed and heated to a pressure of 103,4 kPa. The extraction was carried out in intervals from 0,5 to 3,5 h according to the experimental design. Finally, the employed solvent was released using the lid valve, and it was recovered from the condensation for later use. The lid was removed, as well as the sample holder from inside the pressure system. The extracted fat was deposited in a beaker and left to cool for 30 min in order to determine its mass.
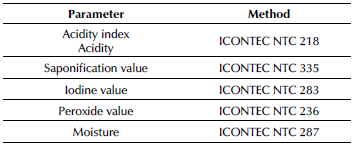
Chemical analysis of extracted fats: The chemical properties were determined through the methodologies described in Table 2.
Table 2 Chemical properties of the extracted fat
Note: NTC: Colombian technical standard
Source: Authors
Obtaining biodiesel from the extracted fat: In a round-bottom flask equipped with a condenser, 30 g of fat were heated to a temperature of 110 °C for 10 min. The reaction mixture was allowed to cool until it reached 100 °C, and then 40 mL of a solution composed of 0,5 g of potassium hydroxide (solid 85%) dissolved in 40 mL of methanol was added (Kubendran et al., 2017). Finally, the reaction was continued at reflux, with constant stirring for 2 hours, according to the methods proposed by Bhatti et al. (2008), Jain et al. (2011), and Mata et al. (2011). The aforementioned amount of methanol is necessary (it was experimentally determined), considering that tallow is a residue from a biological source (variable composition) that does not allow accurately establishing a stoichiometric relationship.
Biodiesel cleaning: The reaction mixture was taken to a separatory funnel and left to stand for 3 h at room tem perature. The upper part was separated (biodiesel) and washed with distilled water at 85 °C until the pH was 7. It was then dried with 1,0 g of sodium sulfate anhydrous, shaken for 60 min, and then filtered and stored in amber glass containers (Mata et al., 2011). The physicochemical properties of the obtained product were then determined as follows: density using the ASTM D1298 method and the kinematic viscosity using the ASTM D 445 method, according to research reported by Srinivasan et al. (2020), and the acid number using NTC 218 of ICONTEC (Colombian Institute of Technical Standards). The materials and reagents are specified in each one of the applied methodologies.
Chromatographic characterization of biodiesel: The characterization of the produced biodiesel was carried out using a Shimadzu GCMS-QP2010S gas chromatograph/ mass spectrometer (Kyoto, Japan). 200 μL of biodiesel were dissolved in 2 mL of hexane, and an Agilent DB-5MS chromatographic column (Santa Clara, CA, USA) (30 m x 0,25 mm x 0,25 pm) was used with the following temperature program: 40 °C initial temperature; increase to 300 °C for 5 min at 10 °C /min; split injector 1:50 at 250 °C; and a mass detector at 300 °C. The amount of sample injected into the equipment was 1,0 μL.
Results and discussion
Sample treatment: Through the chemical treatment of tallow, changes in its organoleptic properties were observed. Due to the presence of calcium hydroxide and impurities that adhere to the samples in the peeling process, such as sand, stone, and vegetal material, a variation in the grey color of the samples was noted. Finally, tallow acquired a white coloration, the unpleasant odor disappeared, and the material had a clear and clean appearance.
Fat extraction with pressurized solvent: The central composite experimental 22 design + star points allowed for the identification of the factors that impact fat extraction in greater magnitudes. The results of the 16 experiments are presented in Table 3.
According to an ANOVA conducted on the complete factorial design, it could be determined that the solvent is the most relevant factor in fat extraction, with a p-value of 0,0006 less than 0,05 at a significance level of 95%, with an R2 value of 90,53% and an adjusted R2 of 76,33%, i.e., greater than 70%, which indicates that the variability in the percentage of fat recovery is explained in great magnitude by the factors studied. Therefore, an adequate estimation of the regression coefficients for the adjusted statistical model and the regression equation can be made in order to predict the percentage of fat recovered by varying the experimental factors, as shown in Equation (1).
where % RF is the percentage of fat recovery, A is the type of solvent, B is the time, C is the quantity of solvent, and AA, AB, AC, BB, BC, and CC are the interactions between the factors.
The response surface plot that demonstrates the adjusted model determined by Equation (1) is depicted in Figure 2.
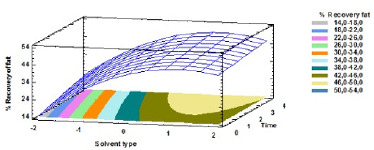
Source: Authors
Figure 2 Response surface plot of interaction between solvent type and extraction time
It is observed that, at times greater than 1 h and when the level-1 solvent (gasoline) is used, the percentages of recovery reach up to 50%.
Table 3 Central composite experimental 22 design + star points
| Type of solvent | Time (hours) | Amount of solvent (mL) | Fat recovery (%) |
|---|---|---|---|
| -1 | 3 | 150 | 36,91 |
| 1 | 3 | 150 | 51,65 |
| -2 | 2 | 100 | 28,34 |
| 0 | 2 | 175 | 46,35 |
| -1 | 1 | 50 | 33,67 |
| 1 | 3 | 50 | 49,81 |
| 1 | 1 | 50 | 48,24 |
| -1 | 1 | 150 | 30,39 |
| 0 | 0.5 | 100 | 39,57 |
| 1 | 1 | 150 | 49,17 |
| 0 | 3.5 | 100 | 46,86 |
| 0 | 2 | 100 | 44,08 |
| -1 | 3 | 50 | 33,45 |
| 0 | 2 | 100 | 44,08 |
| 2 | 2 | 100 | 40,98 |
| 0 | 2 | 25 | 42,17 |
Source: Authors
Commercial gasoline was the solvent that extracted the highest amount of grease, reaching 51,50% in a 3 h period, using 150 mL of solvent and the pressurized extraction technique with solvents in liquid-gas equilibrium, as described in this work. These results are related to the mixture of hydrocarbons present in gasoline (Hua et al., 2018) which provide a lipophilic and apolar environment that favors fat recovery. These values are higher than those obtained with other techniques that have been used for the extraction of fat in this type of waste, using hexane and soxhlet equipment and achieving fat recovery values of up to 11,5%. It is clear that the proposed method has the following advantages: small amounts of solvent are required, commercial gasoline is inexpensive, it has short extraction times, and the solvent used is 70% recovered and can be reused for these same purposes. The risks at work using gasoline (as it is highly flammable and hazardous) are reduced by controlling the experimental conditions of extraction. At the end of the processes and through gas chromatography, it was possible to demonstrate that tallow was obtained without any contaminant, since there is no interference in chemical characterization.
Characterization of the physical and chemical properties of extracted fat: It was observed that the fat is yellow and has a smooth texture, its melting point falls in a range between 38-39 °C, the density is 0,8932 g / cm3, and its dynamic viscosity is 6,93 cP at 60 °C. Table 4 shows the chemical properties observed.
Table 4 Chemical properties of extracted fat
| Parameter | Method | Units | Results |
|---|---|---|---|
| Acidity index Acidity | ICONTEC NTC 218 | mg KOH/g % oleic acid | 1,64 0,82 |
| Saponification value | ICONTEC NTC 335 | mg KOH /g of fat | 188,43 |
| Iodine value | ICONTEC NTC 283 | g I2/100 g | 33,21 |
| Peroxide value | ICONTEC NTC 236 | meq O2/Kg | 5,47 |
Source: Authors
The acidity, saponification, and acidity indices indicate that the degradation of the fat is low, since it contains a low amount of free fatty acids, favoring the extraction of biodiesel. In the transesterification reaction, there are no secondary reactions that alter the composition of the biofuel. The iodine value indicates the low content of unsaturated fatty acids, which confers a great stability of the fat to the oxidative processes, as well as to the mixture of methyl esters present in the biodiesel (de Freitas et al., 2019). The peroxide value indicates the stability of the fat once extracted, and the low content of water remaining in the fat is due to the implemented extraction system, which allows for water to be eliminated in the form of steam through the upper valve (Rahman et al., 2015), as shown in Figure 1a.
Characteristics of the biodiesel: Biodiesel is a slightly viscous liquid with a pleasant aroma, which is a characteristic of esters, with a translucent yellow coloration. When in contact with the flame, it is consumed in a controlled manner, and no sudden reaction to heat is observed. Its density is 880 kg/m3, and it has an acid number of 0,078 mg KOH/g and a viscosity of 3,25 mm2/s at 40 °C (Srinivasan et al., 2020). These values fit the range proposed by the international norms, i.e., ASTM D6751 (ASTM International, 2013), as well as by European standards (EN 14214) (European Committee for Standardization, 2003), which regulate the properties of biofuels. According to the chromatographic analysis (Figure 3), the main components of the methyl esters were palmitic and oleic acids, with 20,10% and 15,7%, respectively (Table 5). These values are confirmed by Sandhya et al. (2016) with values of 46,60% for palmitic acid and 32,2% for oleic acid; as well as by Moraes et al. (2008), who reported values of 26,18% for palmitic acid and 30,09% for oleic acid.
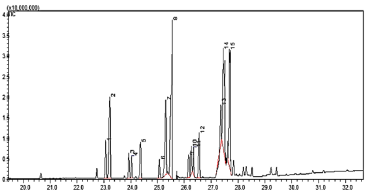
Source: Authors
Figure 3 TIC Chromatogram of methyl esters of acids (biodiesel): 1) miristoleic; 2) miristic; 3) decanoic; 4) arachidic; 5) pentanoic; 6) hexadecanoic; 7) palmitoleic; 8) palmitic; 9) isomargaric; 10) isoheptadecanoic; 11) 8-octanoic (2-methylcyclopropyl); 12) heptadecanoic; 13) elaidic; 14) oleic; 15) stearic
Economic feasibility: 50% of tallow is composed of fat. Approximately 800 mL of biodiesel are produced from each kg of tallow. With 60 tons being produced in the municipality of Belén, approximately 24 000 liters of biodiesel per month can be produced. This amount of biodiesel can be an alternative for the energy needs of the leather production plants, or it can be sold to generate new economic resources. Although Belén is a township with an industrial inclination located in the southwest of Colombia, the applied technology shows low levels of productivity. The leather produced in this industrial area is not competitive, and the profitability of the industry is low. The main limitation to access new markets is the environmental impact caused by solid and liquid waste, so it is necessary to open new production lines such as biodiesel produced from fat contained in tallow. This technology will allow improving profitability while reducing the negative environmental impact of tanneries (Nandhini et al., 2022).
Table 5 Methyl ester components of the biodiesel produced
| Peak no. | Fatty acid | Methyl esters (%) |
|---|---|---|
| 1 | Miristoleic acid | 3,40 |
| 2 | Miristic acid | 11,20 |
| 3 | Decanoic acid | 2,35 |
| 4 | Arachidic acid | 2,20 |
| 5 | Pentanoic acid | 3,60 |
| 6 | Hexadecanoic acid | 1,90 |
| 7 | Palmitoleic acid | 10,00 |
| 8 | Palmitic acid | 20,10 |
| 9 | Isomargaric acid | 2,20 |
| 10 | Isoheptadecanoic acid | 3,20 |
| 11 | 8- (2-methylcyclopropyl) octanoic acid | 2,75 |
| 12 | Heptadecanoic acid | 5,10 |
| 13 | Elaidic acid | 5,90 |
| 14 | Oleic acid | 15,70 |
| 15 | Stearic acid | 10,60 |
Source: Authors
Conclusions
The extraction technique presented in this work, which incorporates the use of a pressurized solvent, proved to be more efficient, with a fat recovery percentage of 51%, short extraction times, and the use of small amounts of solvent in comparison to conventional techniques such Soxhlet extraction. This results in lower production costs of biodiesel from tallow. It was observed that commercial gasoline is a good solvent for the extraction of fat, and it is easy to acquire and relatively inexpensive. The characteristics of the biodiesel obtained from tallow using the extraction technique constitute a novelty of this work.
The physical and chemical properties of the extracted fat indicate that it is an excellent raw material for the production of biodiesel. In the transesterification process, there are no secondary reactions derived from fat degradation processes, thus allowing to obtain high-quality biodiesel. The biodiesel has good characteristics that are in the ranges reported by technical standards ASTM D6751 (ASTM International, 2013) and EN14214, and it shows good a performance, considering that, from 30 g of raw material used in the transesterification reaction, 22,09 g of biodiesel were extracted.
After extracting the fat from the tallow, a solid residue remains, which is mainly collagen. Studies should be carried out to propose its application as a filling material in filters to remove heavy metals from wastewater.