Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.69 Medellín Oct./Dec. 2013
ARTÍCULO ORIGINAL
Lipid Vesicle Detection using ISFET devices
Detección de vesículas lipídicas con ISFETS
Juan Carlos Ortiz Pereira1, Alba Graciela Ávila1, Juan Carlo Briceño2*
1Departamento de Ingeniería Eléctrica y Electrónica, Universidad de los Andes, Carrera 1 N° 19A 40. Bogotá, Colombia.
2Departamento de Ingeniería Mecánica. Universidad de los Andes, Carrera 1 N° 19A 40. Bogotá, Colombia.
*Autor de correspondencia: teléfono: + 57 + 1 + 339 49 49, correo electrónico: jbriceno@uniandes.edu.co. (J. Briceño)
(Recibido el 10 de abril de 2012. Aceptado el 9 de octubre de 2013)
Abstract
The Ion Sensitive Field Effect (ISFET) Transistors are electronic devices widely used for detecting, ion concentrations, trapped charge or charge decay processes. This article reports on the electrical characterization of ISFETs exposed to buffer solutions sets at pH 4, 5, 7, and 10. When operated in their saturation regime ISFETs act as local charge detectors. This capability is applied to detect lipid vesicles. Lipid vesicles have promising applications in drug delivery, and their accurate detection can be important in understanding their interaction with living systems.
Keywords: ISFET, detection, lipid vesicle
Resumen
Los transistores de efecto campo sensibles a iones (ISFET) son dispositivos electrónicos con un amplio uso para la detección de concentraciones de iones, cargas confinadas o procesos de decaimiento de carga. Este articulo reporta la caracterización eléctrica de ISFET expuestos a soluciones buffer de pH 4, 5, 7 y 10. Cuando los ISFET operan en región de saturación funcionan como detectores de carga localizada. Esta habilidad se usa en este trabajo para detectar vesículas lipídicas. Las vesículas lipídicas tienen una amplia aplicación en el trasporte de medicamentos, y por lo tanto su detección puede ser importante para entender su interacción con otros sistemas vivos.
Palabras clave: ISFET, detección, vesículas lipídicas
Introduction
The Ion Sensitive Field effect was first introduced in 1970 for measuring ion activities in electrochemical and biological environments [1]. The ISFET is a metal-oxide-semiconductor field effect transistor (MOSFET)-based device, without a gate metal. Generally the free gate- insulator area is exposed to an ion concentration; buffer solution or electrolyte solution to replace the metallic gate. The current between the source and drain electrodes of the device senses the local charge variations on the expose gate- insulator area. The first application of an ISFET was operation in liquids, as a pH-sensor [2]. As an extension of MOSFETS, ISFETS are also fabricated on Silicon substrates which have brought the attention of several application areas due to its compatibility with standard micro fabrication process, its potential integration and its miniaturization towards and integrated Microsystems for biomedical applications [3, 4].
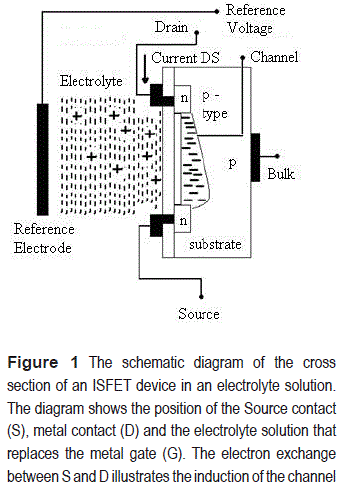
The ISFETs generally operate as an enhancement MOSFET. For an n-channel MOSFET, the substrate is p-type doped with negative impurities at both sides of the gate region; these are the n-type silicon regions for the source (S) and the drain (D). When positive voltage is applied on the gate region, it repels the proximal positive charges in the substrate and attracts electrons in the channel region, inducing a channel between the D and S. The opening of the ISFET channel is therefore defined by the concentration of free cations in the electrolyte solution. The channel width is controlled by the positive charge concentration. In order to use the ISFET device as a sensor to detect variations in cation concentration, the channel has to be initially induced by using a reference electrode to keep the solution at reference potential, see figure 1.
Operating the ISFET at the saturation region, variations in the drain-source current are directly related to the charge density on the channel [5]. This charge could be a consequence of the presence of charged vesicles or particles close to the detection area. In this paper, two applications for the ISFETs are illustrated: as pH and vesicles detectors. The current measurements allow us to estimate the charge of the detected suspended vesicle solution, opening possibilities for its electrical characterization. Measuring the charge is a challenge in the context of the application of vesicles in drug delivery.
Experimental
The process for lipid vesicle or Giant Unilamellar Vesicle (GUV) electroformation, the ISFET parameters and the ISFETs experimental set-up for pH and vesicle detection are presented below.
Lipid vesicles electroformations
GUVs were obtained by the electroformation method, first described in relation to a home built Teflon chamber using Pt electrodes to avoid oxidation [6]. Three (3) μl of a 0,2 mg/ml lipid/ chloroform mixture labeled with 1,0mol% of 1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4- yl)amino]hexanoyl]-sn-glycero-3-phosphocholine (NBD-PE) were deposited in each Pt electrode (the NBD-PE were purchased from Avanti Polar Lipids (Alabaster, AL) ). The sample was then placed overnight under vacuum to remove traces of the organic solvent. Sufficient amounts of 200m0sM sucrose solution (17,5 MΩ; Millipore, Billerica, MA) were then added to the Pt electrodes to cover them completely, and a low frequency AC field was applied using a function generator (sine wave at a of frequency of10 Hz with 2Vpp amplitude) for 1-2 h. As a result, GUVs with diameters around 30 to 100 mm are electroformed.
After vesicle formation, the AC field was turned off and the vesicles were transferred to an iso- osmolar glucose solution in a special chamber over the ISFET device (270 μl of glucose + 90 μl of the GUVs in sucrose). This new solution helps the GUVs settle on the chamber bottom surface.
ISFETs
The ISFETs used in this work were fabricated at the National Center of Microelectronics in Spain (CNM). The ISFETS and its connections are mounted on a printed circuit board (PCB) and coated with an epoxy resin (EPO-TEK H77, Epoxy Technology). The ISFET (fabricated into 3mm x 3 mm chips) is attached to one end of the PCB and the sensible channel area is free of epoxy coating (5000 μm2) to be exposed to the buffer and suspended vesicles solution.
pH-detection set-up
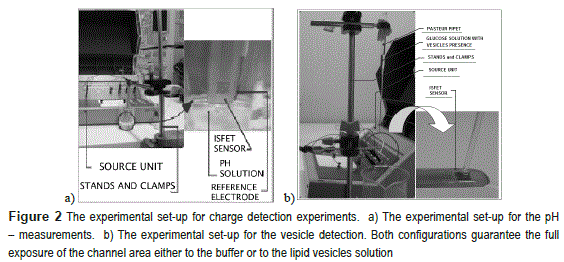
The gating properties of each ISFET device were characterized at four distinct buffer solutions. figure 2a shows the experimental set-up for the ISFET characterization at 25 ml of pH solutions: 4, 5, 7 and 10, these values are verified with a calibrated pH meter Oakton pc 510. For the pH detection, a clamp was used to immerse the ISFET vertically into different solutions. With the source unit and the reference electrode a DC voltages were applied to induce the channel.
Vesicle-detection set-up
Figure 2b shows the experimental set-up for the vesicle experiment. The clamp was used to horizontally hold both the ISFET and the Pasteur pipette. The pipette contains 270μ1 of glucose (0.5 Molar) and 90μl of the GUVs in sucrose. An external electrode (not visible in the figure) was used to bias the ISFET before the injection of vesicles or buffer solutions. This bias controls the formation of the channel and it also allows controlling the operation region of the ISFET.
A critical step in vesicle detection experiments is ensuring that the channel area is cleaned from any organic residues. The drain-source current drops 50% of its original value when organic residues remained on channel, compromising the ISFET measurements. The ISFETs then were cleaned according to the following protocol: first it was immersed in ethanol for 30 minutes or 2 hours depending on previous residual conditions and subsequently, it was washed in DI water and dried with compressed air. The cleaning process was verified through visual inspection.
Results
Previous to the vesicle detection, the input and output I-V characteristic curves of the source common ISFET configuration are measured at different buffer solutions. Forcing the ISFET to operate in the saturation region, the current varied depending on the pH-solution, which generated a calibration curve. The calibration curve allowed us to determine the pH value of a given vesicle solution and therefore, to estimate its charge density.
Ph-detection
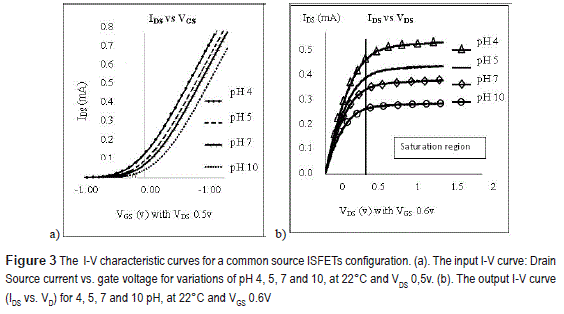
Figure 3 shows the current-voltage (I-V) input and output characteristics at 22°C, determined by sweeping the drain (VD)-reference electrode (Vr) voltages independently, using a source/monitor unit E5287A: VD from 0v to 1,5v, and Vr from -1,0v to 1,2v. These curves provide information of the threshold voltage for the channel induction (see figure 3a) and subsequently the VDS at which the IDS current is independent of voltage variations (see figure 3b, the vertical line indicates the saturating voltage (VDSAT)): VDs=>0.5 V.
Figure 3b shows that the IDS current increases with the acidic solutions (pH <7) and it decreases with basic solutions (pH>7). Since pH is defined as a negative decimal logarithm of the hydrogen (H+) and hydroxide ions (OH-) activity in a solution in units of molar concentration at pH 4 and pH 10, the concentrations of H+ and OH- are molar and respectively. Based on this concentration, Faraday constant and the volume of the solution, charge density was estimated as discussed in more detail below [7, 8].
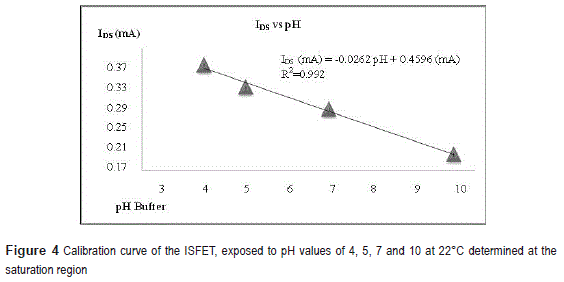
In figure 4 the IDS as a function of pH buffer determined from figure 3b, is presented, the ISFET's sensitivity is then determined as 26,2 mA/pH.
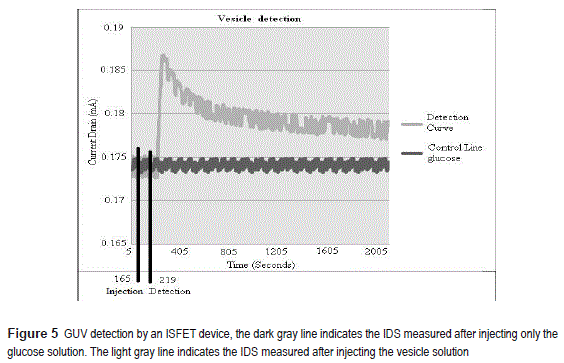
Vesicle detection
The detection of vesicles using the set-up introduced in section 2.4, is described in detail here.
The ISFET is first forced to operate in the saturation region by applying VDS =0,5 V and VGS =0,51 V at 22oC. Subsequently, the IDS was registered as function of time for two solution injection conditions: (A) adding only glucose solution, (B) adding glucose + a lipid vesicle solution into the pipette, see figure 5. In case (A), the IDS measured is constant (0,174 mA), on the contrary, for case B, the current presents a peak and it increases up to 0,187 mA. This single peak is detected 55 seconds after the vesicles were injected into the column. Using the fitting equation of figure 4, a pH of 10.4 was estimated; hence, the vesicle seems to have a negative charge density.
Given that the pH from the IDS measurements can be determined, the definition of pH can be used to determine the molar concentration of the hydroxyl ions (OH):; from where OH- concentration can be calculated as: and expressing the concentration in mol units (in a total volume of solution of 360μL), we obtained ~ mol. Transforming the moles of electrons into coulombs of charge, a charge of is estimated for the suspended vesicle solution.
Conclusions
An ISFET device has been characterized as a function of pH. The operation regions at different pH values can be used for suspended vesicle detection. In the experiments described here the ISFET cleaning protocols have been determined avoiding the compromising of the devises when reused in multiple experiments.
Vesicle detection on the sensing ISFET area was achieved by monitoring the current variations operating in the saturation region during the injection of the suspended vesicle solution. This detection approach could also be useful for the study of vesicle charge degradation by monitoring the current variations in periods of days. ISFETs proved to be a useful device with lower instrumentation complexity and practical trigger point to run a quick test of charge vesicle presence in the solutions.
Acknowledgements
We are grateful to Prof Chad Leidy, Hector Jackson Ocampo Ariza and Ivan Adolfo Rey Suarez members of the Biophysics Group at Andes University for their assistance providing the vesicle samples and for their critical feedback and valuable discussions. This work was supported by a Research grant from the Engineering Faculty of the Universidad de los Andes under a grant 0012007.
References
1. P. Bergveld. ''Development of an ion-sensitive solid-state device for neuro physical measurements, short communication''. IEEE Trans. Bio-Med. Eng.Vol. 17. 1970. pp. 70-71. [ Links ]
2. Y. Ghallab, W. Badawy, K.V.I.S. Kaler. A novel pH sensor using differential ISFET current mode read-out circuit. International Conference on MEMS, NANO and Smart Systems. Banff, Canada. 2003. pp. 255-258. [ Links ]
3. V. Chodavarapu, A. Titus, A. CartWrright. CMOS ISFET Microsystem for Biomedical Applications. In procceding of: Irvine, CA, US. 2005. pp 109-112. [ Links ]
4. D. Schaffhauser, M. Patti, T. Goda, Y. Miyahara, I. Forster. ''An Integrated Field-Effect Microdevice for Monitoring Membrane Transport in Xenopus laevis Oocytes via Lateral Proton Diffusion''. PLoS ONE. Vol. 7. 2012. pp. 1-8. [ Links ]
5. G. Grieshaber, R. MacKenzie, J. Vo ros E. Reimhult. ''Electrochemical Biosensors-sensors principles and architectures''. Sensors. Vol. 8. 2008. pp. 1400-1458. [ Links ]
6. M. Angelova, S. Soleau, P. Meleard, J. Faucon, P. Bothorel. ''Preparation of giant vesicles by external a.c. electric fields. Kinetics and applications''. Prog. ColloidPolym. Sci. Vol. 89. 1992. pp. 127-131. [ Links ]
7. C. Cane, I. Gracia, A. Merlos, M. Lozano, E. Lora- Tamayo, J. Esteve. Compatibility of ISFET and CMOS technologies for smart sensors. Proc. Int. Conf. Solid-State Sensors and Actuators (Transducers '91). San Francisco, USA. 1991. pp. 225-228. [ Links ]
8. A. McNaught, A. Wilkison. Compendium of Chemical Terminology. 2nd ed. The ''Gold Book'', Blackwell Scientific Publications. Oxford, England. 1997. pp. 248 - 552. [ Links ]