Introduction
The periodontium is a specialized connective tissue that is constituted by the gum, the periodontal ligament, the cement and the alveolar bone. Its main function is to provide support and support to the tooth in its alveolus 1 The teeth are in contact with the junctional epithelium, a non-keratinized stratified squamous epithelium that allows the migration of polymorphonuclear neutrophils (PMN) as a defense mechanism 2.
The periodontal tissue is affected by periodontal diseases and conditions that includes various pathologies including periodontitis whose classification and definition were updated by the American Academy of Periodontics (AAP) and the European Federation of Periodontics (EFP) in 2017 3. Periodontitis is a chronic inflammatory disease of high prevalence, affecting 11.2% of adults in the world, which represents the sixth most common human disease 4. In Colombia, the majority of the population (61.8%) between 18 and 79 years old evidences periodontitis in its different degrees of severity 5.
Periodontitis has been linked to obesity, type 2 diabetes mellitus 6, type 1 diabetes mellitus 7, cardiovascular disease and coronary heart disease 8.
There is evidence of a positive association between periodontitis and oxidative stress 9. During periodontitis, ROS are released from PMN in response to invading microorganisms, these ROS are responsible for oxidative stress and much of the tissue damage during infection 10. This association between oxidative stress and pathophysiology and tissue destruction in periodontitis has been subject to observational, experimental and systematic reviews, but these are not enough and the results are inconclusive 11) and the signaling pathways are not well established, so more research in this area is necessary to a comprehensive understanding of periodontitis 12. A better understanding of this association can give us a more established view of the pathogenesis of periodontitis, and therefore improve therapeutic strategies.
The objective of this review is to describe the role played by ROS and oxidative stress in the development and evolution of inflammation and tissue injury during periodontitis. In this review, the following terms are defined and explained: periodontitis, free radicals, ROS, oxidative stress, antioxidant systems and the mechanism of action of reactive oxygen species and oxidative stress in tissue destruction in periodontitis, including signaling pathways which regulates ROS to produce tissue injury in the periodontium
Materials and methods
Type of study: Review article
Topic: ROS, oxidative stress and its relationship with the pathophysiology of periodontitis.
Inclusion criteria: research published during published from January 1, 2007 to December 31, 2018 full text, clinical trials, cross-sectional studies, longitudinal studies, observational studies, cross-sectional studies, cases and controls, review articles, systematic reviews, epidemiological reports, articles related to the current classification of periodontal diseases and conditions, articles on the global burden of periodontal diseases, book chapters and in vitro and in vivo studies. An idiomatic restriction was applied, including only works published in English and in international journals, except the national epidemiological report (ENSAB IV).
Exclusion criteria: letters to the director, newspapers, conferences, news, comments, case reports, symposia, compendiums, pilot studies, expert opinions and illustrated essays.
Search strategies
Initially a preliminary search was conducted to estimate the amount of information published on the subject under study, identify the terms that will be used in the search and the most appropriate databases. A systematic search was then carried out in the following databases: PubMed, ScienceDirect, Wiley Online Library, Springer, Plos one, Nature, Sage journals, Hindawi and Taylor & Francis Online. Descriptors of such as "ROS", "oxidative stress", "antioxidant", "chronic periodontitis" and "periodontal disease" "periodontitis" were used and related to each other and with free terms. This search was executed during June 1, 2017 to December 31, 2018.
The authors of this document made critical readings of the articles for their selection, extraction and data analysis. The selected articles were classified and sorted according to their type and according to the descriptors previously mentioned. In total, 68 scientific publications were selected.
Results
Of the 68 selected publications that met the inclusion criteria, 32 are review articles, 11 are case-control investigations, 7 are in vitro studies, 3 are clinical trials, 2 are systematic reviews, 3 are cross-sectional studies, 2 are chapters of books, 2 are articles developed in the context of the 2017 world workshop on the current classification of periodontal and peri-implant diseases and conditions, 1 article on the impact of the global burden of periodontal diseases developed in the context of the Milan World Exposition 2015 "Feeding the planet, energy for life", 1 longitudinal study, 1 observational study, 2 experimental study with in vitro and in vivo models and 1 epidemiological report.
Periodontitis
Periodontitis is an inflammatory disease that causes loss of the periodontal junction in which the host and the microbiota are associated. During this disease, host derived proteinases are activated, which allow the loss of marginal fibers of the periodontal ligament, the apical migration of the binding epithelium and the apical propagation of the bacterial biofilm in the tooth root 13. This disease produces destruction of the soft and hard tissues that support the tooth 14, mobility of the teeth and ultimately the loss of the same 15. The diagnosis and classification of this pathology is carried out by traditional clinical measurements such as depth of the periodontal pocket, bleeding on probing (BOP), clinical attachment loss (CAL) and radiographic bone loss 16-17.
The primary etiological factor of periodontitis are pathogenic microorganisms, while the immune response affects the progression of the disease 18, which arises from excessive inflammatory responses resulting from complex interactions between the host and the subgingival dysbiotic microbiota that involve elements both innate and adaptive immunity 19. Periodontopathogenic bacteria include: Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Porphyromonas gingivalis (P. gingivalis), Tannerella forsythia (T. forsythia), Treponema denticola (T. denticola) and Prevotella intermedia (P. intermedia) 20, as part of aggressive bacteria classified in colors by Socransky 21.
Periodontitis is characterized among many other aspects, by the production of prostaglandins E2 (PGE2), proinflammatory cytokines such as interleukin 1 (IL1), interleukin 6 (IL6), tumor necrosis factor α (TNFα) and ROS. These factors promote tissue destruction by inhibiting collagen synthesis, activating matrix metalloproteinases (MMPs), blocking the activity of tissue inhibitors of matrix metalloproteinases (TIMP), and at the same time stimulating bone resorption of alveolar bone 22.
This pathology is associated with hyper-reactive neutrophils with an increased production of ROS in response to the stimulation of Fc-gamma receptors (FcγR) 23, tolllike receptors (TLR) and cytokine receptors 24, resulting in periodontal tissue injury and activation of macrophages, neutrophils and fibroblasts to generate more ROS 22.
Free radicals and reactive oxygen species
Free radicals are atoms or groups of atoms that have an unpaired electron in their outermost orbit, which gives it high instability, reactivity and an enormous capacity to combine nonspecifically with biomolecules, producing different radical and non-radical species, which are generally harmful to humans, since they can alter the configuration of molecular structures, leading to damage or molecular and tissue instability 25. When the free radical subtracts the electron it needs, a redox reaction takes place, in which the free radical is reduced and the stable molecule that has lost the electron is oxidized, becoming a free radical and thus initiating a chain reaction 26. As a product of metabolism, different types of free radicals are generated, such as: ROS and reactive nitrogen species (RNS) 25.
ROS are broadly defined as chemically reactive oxygen-containing molecules 27. They can be classified into free radicals and non-radical oxygen species involved in the production of oxygen radicals. Free radicals include the superoxide anion (O -), hydroxyl (OH-), hydroperoxyl (HOO-), peroxyl (ROO-). Singlet oxygen (1O2 ), hypochlorous acid (HOCl) and hydrogen peroxide (H2O2) are non-radical oxygen species 26. El OH-, O2 - and H2O2-are of greater physiological importance 28.
Sources and formation of reactive oxygen species
The cells of the body are exposed to oxidants from endogenous and exogenous sources. Exogenous sources include heat, trauma, ionizing radiation, UV radiation, ozone, smoking, infection and metabolism of a broad spectrum of drugs and xenobiotic. Endogenous sources are mainly byproducts of metabolism by functional generation by host defense cells (phagocytes) and cells of connective tissues 22-29.
The main endogenous source of ROS is the electron transport chain (ETC). From the mitochondrial respiratory complexes I and III in the ETC some electrons escape and react with oxygen to generate O - (30), which is transferred to the mitochondrial matrix through the inner mitochondrial membrane. Subsequently, by action of superoxide dismutase 2 (SOD2), O2 - becomes H2 O2 . O2 - is also produced in the cytosol by enzymatic reactions involving NADPH oxidases (NOX), xanthine oxidase, arachidonic acid, among others 27. H2 O2 is a much more stable molecule O2 -, capable of diffusing through biological membranes. In the presence of metal cations, such as iron or copper, H O is converted to OH through the Fenton reaction 31-32. The O2 H2- is extremely unstable and reactive; with high oxidizing power, it oxidizes lipids, proteins and DNA indiscriminately, resulting in damage or genomic instability 26.
Oxidative stress
When the production of ROS and RNS increases in such a way that the antioxidant response cannot restore the system, oxidative stress occurs 10. The term oxidative stress was defined in 1985 by Helmut Sies as a disturbance in the prooxidant-antioxidant balance in favor of the former, giving rise to potential damage 33-34 . Nowadays, oxidative stress is defined as a persistent imbalance between the production of highly reactive molecular species (oxidants) and antioxidants in favor of oxidants, which leads to an interruption of redox signaling and control and/or molecular damage 35. Oxidative stress can be divided into four ranges: basal oxidative stress, low intensity, intermediate and intense. Probably, low intensity oxidative stress is detected by the Nrf2/Keap system, which is activated by minimal amounts of ROS 36. The nuclear transcription factor NF-E2 related to factor 2 (Nrf2) translocates to the nucleus where it interacts with antioxidant response elements (ARE) in promoters of target genes that encode antioxidant defense enzymes 37. NF-κB, activating protein 1 (AP-1) and MAP kinases are involved in intermediate intensity oxidative stress through the expression of antioxidant enzymes and certain genes of inflammation and reprogramming of general cellular functions. Apoptosis or necrosis occurs in response to intense oxidative stress 36).
Antioxidant systems
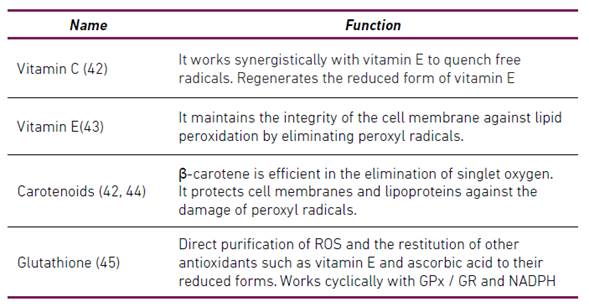
Antioxidants are substances that are present at low concentrations and retard or significantly prevent the oxidation of an oxidizable substrate 38. Antioxidants can be classified according to their mode of action in preventive antioxidants and antioxidants that break chains (Scavenging) 34. Antioxidants have also been classified as enzymatic and non-enzymatic 28-30-39-40. Table 1 summarizes some enzymatic antioxidants and Table 2 summarizes some non-enzymatic antioxidants.
Table 1 Enzymatic Antioxidants
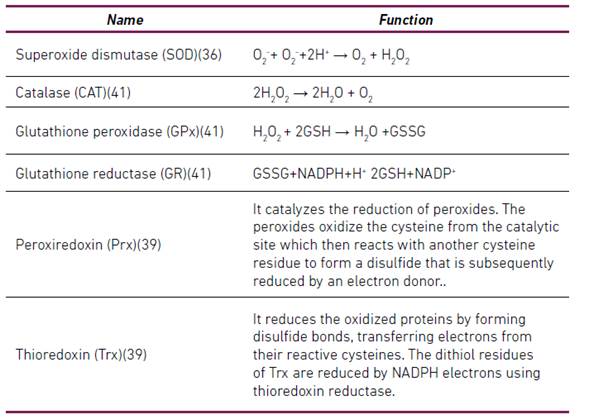
*GSH (reduced glutathione), GSSG (glutathione disulfide), H2O2 (hydrogen peroxide), NADP+ (nicotinamide-adenine-dinucleotide-phosphate), NADPH (reduced nicotinamide-adenine-dinucleotide-phosphate), O2- (superoxide anion)
Mechanism of action of reactive oxygen species and oxidative stress in tissue destruction in periodontitis
Tissue destruction in periodontitis is considered to be the result of an excessive inflammatory response to the subgingival biofilm 12, since cellular components such as lipopolysaccharides (LPS) and the DNA of periodontopathogenic bacteria cause the activation of signaling pathways related to inflammation in gingival fibroblasts and the production of inflammatory cytokines such as interleukin-8 and TNF-α. Similarly, hyperreactive recruitment and activation of PMNs that produce ROS in response of the host to pathogens occurs 22. In this sense, neutrophils produce O2 - by respiratory burst with the participation of the NOX enzyme. In turn O2 - can be released from the phagosomal environment to the extracellular to become ROS such as OH-, 1O2, HOCl and H2O245.
The release of ROS plays a critical role in the destruction of tissue in periodontitis 12, these in excess cannot be balanced by the antioxidant defense system, which leads to oxidative stress and tissue damage in the periodontium 45. Oxidative stress can cause damage directly to the periodontal tissue and indirectly to it by activating cell signaling pathways related to inflammation, apoptosis and other factors 46. Direct tissue damage caused by ROS includes cytotoxic effects such as lipid and phospholipid peroxidation, oxidative damage to proteins and DNA, interfering with cell growth and cell cycle progression, inducing apoptosis of gingival fibroblasts and causing matrix degradation extracellular (MEC) through MMP induction and glycosaminoglycan breakdown 12, cell membrane destruction, protein denaturation and enzymatic inactivation, mitochondrial lesions and more ROS production 46. Protein damage involves folding or unfolding proteins, protein fragmentation and polymerization reactions, protein radical formation, formation of protein carbonylation products. DNA damage causes chain breaks, base mutations, guanine to 8-hydroxyguanine conversion, deletions, insertions and sequence amplification 22,29.
Tissue destruction can be assessed by measuring biomarkers malondialdehyde (MDA), 8-hydroxysoxyguanosine (8-OHdG), carbonyl proteins (PC), total antioxidant capacity (TAOC), SOD, among others 45. Patients with periodontitis have higher levels of MDA 47, 8-OHdG 48, PC 49 and lower levels of SOD 50 and TAOC tan healthy controls 51.
Likewise, this damage to periodontal tissue leads to the overproduction of lipid peroxides, inflammatory mediators and oxidized proteins, which further activate macrophages, neutrophils and fibroblasts to generate more ROS and create a vicious circle 22.
Indirect tissue damage caused by ROS occurs through the following mechanisms:
ROS can promote osteoclastogenesis 12.
Activation of NF-κB, initiating a signaling cascade that regulates inflammatory and immune responses.
Activation of JNK, producing cellular apoptosis.
ROS are associated with inflammasome activation resulting in piroptosis.
ROS play a critical role in autophagy. However, there is still no direct evidence to show that the activation or inactivation of autophagy is triggered by the regulation of redox signaling in periodontitis 46.
ROS can indirectly promote osteoclastogenesis because they act as an intracellular signaling molecule during it. Alveolar bone destruction and resorption requires osteoclasts, in turn the generation of osteoclasts requires that NF-κB activating receptor ligand (RANKL) bind to its receptor (RANK) in the marrow macrophages which leads to differentiating them into osteoclasts. Under physiological conditions, RANKL is expressed mainly in mesenchymal cells of the osteoblast lineage, but in periodontitis it is abundantly produced by activated lymphocytes 12).
ROS activate or depress the NF-κB signaling pathway. Some studies have shown that ROS, in particular H2O2, can activate IKK (IkB kinase complex) in some cell types. In contrast, other investigations have shown that H2O2 in some cells can inactivate IKK, in this sense the inhibitory effect can be mediated by the oxidation of IKKβ in cysteine 179 by ROS. It is presumed that NIK, the kinase involved in the non-canonical pathway, is activated by ROS through the inhibition of phosphatases. In addition, the direct oxidation of p50 by ROS inhibits its ability to bind to DNA 52. H2O2 regulates the activation of NF-κB and does so in part through the alternative phosphorylation of IκBα in Tyr42 53. The activation of NF-κB increases the concentration of MMP 22, promotes the expression of proinflammatory cytokines, these factors lead to inflammatory responses and osteoclastic differentiation and thereby the destruction of periodontal tissue 54.
Oxidative stress can also activate JNK signaling during periodontitis 46. It has been shown that JNK activation induced by ROS occurs through the regulatory kinase of the signal of apoptosis 1 (ASK-1) 55. ASK1 produces the subsequent activation (phosphorylation) of JNK, phosphorylated JNK activates apoptotic signaling either through overexpression of proapoptotic genes by transactivation of specific transcription factors or by directly modulating the activities of pro and antiapoptotic mitochondrial proteins by phosphorylation events 56. In the mitochondria JNK amplifies ROS production generated largely by Complex I 55. JNK can stimulate the release of cytochrome C from the inner mitochondrial membrane through Bid-Bax, promoting the formation of apoptosomes. In another mechanism, JNK can promote the release of Smac/Diablo (Smac) by inactivating the inhibitory complex TRAF2/IAP1 to initiate the activation of caspases 56. One study showed that the activation of JNK by ROS disrupted the periodontal junction epithelium through dissociation of E-cadherin 57.
ROS are involved in the pathogenesis of periodontitis through the activation of the NLRP3 inflammasome pathway 58. In this sense an increase in ROS concentrations activates the protein that interacts with thioredoxin (TXNIP) and this binds with the protein with pyrin 3 domain of the NLR family (NLRP3) activating the NLRP3 inflammasome 59-60, which eventually upon activation of caspase-1 to cleave pro-IL-1β and pro-IL-18 in its active forms of secretion (IL-1β and IL-18) and induce pyroptosis 61-63. Elevated levels of IL-1β and IL-18 may contribute to periodontal destruction and inflammation 64.
ROS can activate or suppress the autophagy process, through the following potential mechanisms:
Phosphorylation of the Bcl-2 apoptosis regulator by JNK in a ROS-dependent manner that leads to the dissociation of Beclin 1 (BECN1) and therefore induces autophagy.
Initiation of the PI3K/AKT pathway, which leads to the activation of the rapamycin target protein in mammalian cells (mTOR), inhibiting autophagy.
ROS excesses produce activation of the AMP-activated protein kinase (AMPK), which phosphorylates the tuberin protein (TSC2) and activates the TSC1/TSC2 complex, thus inhibiting the target protein of rapamycin complex1 (TORC1) and stimulating serine/threonine protein kinase (ULK), which is an important initiator of autophagy.
Activation of the Atg12-Atg5 complex, which promotes the elongation of autophagy 46.
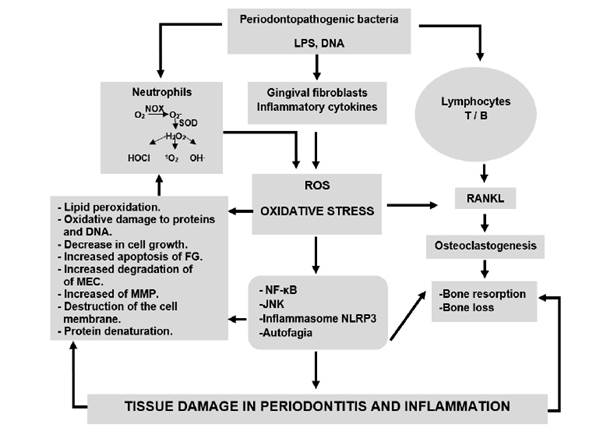
Together, the factors mediated by ROS that cause periodontal destruction directly or indirectly contribute to the development and progression of periodontitis 12,22. Figure 1 summarizes the mechanisms of action of ROS, oxidative stress in tissue destruction in periodontitis.
The periodontopathogenic microbiota can activate immune system cells such as neutrophils, T and B lymphocytes and gingival fibroblasts. Activated neutrophils produce the superoxide anion (O2 -), using the enzyme NADPH-oxidase in the process of respiratory explosion. The O - can be converted by superoxide dismutase or by spontaneous dismutation into hydrogen peroxide (H2O2), which can be converted into other ROS including hypochlorous acid (HOCl), hydroxyl radical (OH-) and singlet oxygen (1O ). An increase in ROS produces oxidative stress, which together can cause direct and indirect damage to periodontal tissue. Direct damage includes lipid peroxidation, oxidative damage to proteins and DNA, decrease in cell growth, increased apoptosis of gingival fibroblasts (FG), increased degradation of the extracellular matrix (MEC), increased matrix metalloproteinases (MMP), destruction of the cell membrane and protein denaturation direct damage includes stimulation of osteoclastogenesis, activation of the pathways signaling nuclear transcription factor kappa B (NF-κB), the N-terminal c-Jun kinase pathway (JNK), activation of Inflammasome NLRP3, activation or inhibition of autophagy. Activated T and B lymphocytes produce RANKL that generates osteoclastogenesis, promoting bone resorption and bone loss. In turn, bacterial cellular components such as DNA and lipopolysaccharides (LPS) stimulate the activation of signaling pathways related to inflammation in gingival fibroblasts and the production of inflammatory cytokines. All these factors play an essential role in the destruction of periodontal tissue and create a vice circle of inflammation that produces even more ROS.
Discussion
The association between oxidative stress and periodontitis has been the subject of review-type investigations, this article has tried to describe and give a possible explanation of this association through the mechanisms of action that allow ROS and oxidative stress to originate and establish periodontal destruction, reflecting that these factors are fundamental in the pathogenesis of periodontitis. Similarly, Wang et al. report that periodontitis is associated with a hyperactivity of peripheral blood neutrophils that are the main source of ROS and oxidative stress is implicated in the pathogenesis of periodontitis 45. In the same way, Dahiya et al., Liu el al. establish that oxidative stress is fundamental in the damage to periodontal tissue that results from interactions between the host and the microbiota due to increased ROS, antioxidant deficiency or by activating signaling pathways sensitive to redox changes and creating a proinflammatory state 22-46. This differs with Tóthová et al. who expressed that despite decades of research the role that oxidative stress plays in periodontitis is unclear 11.
Previously, it was mentioned that ROS cause lipid peroxidation, oxidative damage to proteins and DNA, so research has been carried out that evaluates the different biomarkers of oxidative stress in periodontitis. A widely studied biomarker is MDA, which is a product of lipid peroxidation 14). Some research has studied MDA levels in patients with periodontitis. Trivedi et al. conducted a study in which spectrophotometrically analyzed MDA levels in individuals with type 2 diabetes mellitus with and without periodontitis and periodontally healthy patients with and without type 2 diabetes mellitus, in this study it was shown that MDA levels in both groups of periodontitis were higher than in periodontally healthy groups, concluding that the body's antioxidant mechanisms are partially collapsed due to excessive free radical production during periodontitis 47), which supports the results of Almerich-Silla et al. in which significantly higher levels of MDA are reflected in patients with periodontitis than in healthy controls and gingivitis, demonstrating significant changes in oxidative stress by measuring different markers of oxidative stress that increased with the deterioration of the periodontal state 16. High levels of serum and salivary MDA, without changes in antioxidant status, can cause systemic and local complications in patients with periodontitis 65. On the other hand Baltacıoğlu et al. They reported that there is no significant difference in serum MDA levels, but in salivary of this same biomarker in patients with periodontitis and healthy controls 66.
A biomarker of oxidative damage of DNA that has been studied in periodontitis is 8-OHdG. The results published by Villa-Correa et al. showed higher salivary levels of 8-OHdG in individuals with periodontitis than in healthy controls, this shows that the increase in levels in this biomarker may be a prognostic indicator of periodontal destruction induced by oxidative stress 48. These results are consistent with those presented by Zamora-Pérez et al. with higher levels of salivary 8-OHdG in periodontitis, which indicates a direct relationship with DNA damage 67.
Oxidative damage to proteins can cause their denaturation and therefore the loss of their function 46, one way to estimate this damage is by measuring PC, which is higher in patients with periodontitis, This suggests that there is an increase in oxidative stress during periodontitis 49.
Antioxidants have also been evaluated in patients with periodontitis. Trivedi et al. compared salivary levels of SOD in subjects with periodontitis and healthy, revealing lower measurements of this antioxidant enzyme in the periodontitis group than in the control group, which evidences that oxidative stress plays a fundamental role in the pathogenesis of periodontitis 68. Similarly Canakci et al. support less SOD activity in periodontitis 50.
Conclusion
Oxidative stress (excess ROS) plays a critical role in the development and evolution of tissue injury and periodontal inflammation, which leads to tissue destruction and creation of a proinflammatory state in periodontitis. This is because ROS are capable of stimulating the production of proinflammatory molecules, regulate apoptosis, activate immune cells that produce more ROS, creating a vice circle of inflammation and enhancing tissue injury. In addition, ROS stimulates osteoclastogenesis, producing bone resorption, which would lead to tooth mobility and long-term tooth loss. However, it cannot be inferred whether oxidative stress is a factor prior to the development of periodontitis or if it is only triggered as a result of periodontal inflammation. Therefore, investigations of oxidative damage in this pathology and the development of biomarkers of oxidative stress could be useful for the monitoring and evaluation of periodontal treatment.
On the other hand, observational and experimental research on these topics is required to be more homogeneous in terms of the type of sample and the methods used to estimate and measure the different oxidative stress biomarkers.