Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.3 Bogotá July/Sept. 2020 Epub Mar 01, 2021
https://doi.org/10.22516/25007440.502
Review article
Radiation enteritis. Case report and literature review
1Especialista en Gastroenterología, Pontificia Universidad Javeriana. Jefe de la Unidad de Gastroenterología, Hospital Universitario San Ignacio, Pontificia Universidad Javeriana; Bogotá, Colombia
2Especialista en Gastroenterología, Pontificia Universidad Javeriana. Hospital Universitario San Ignacio, Pontificia Universidad Javeriana; Bogotá, Colombia
Radiation enteritis is a pathology caused by radiation therapy, used to treat radiosensitive tumors. Acute or chronic eneritis may be suspected in the presence of symptoms such as malabsorption or intestinal obstruction, which alter the patients quality of life. The following is the case report of a 67-year-old male patient, who consulted for symptoms of intestinal obstruction, with a history of diffuse type adenocarcinoma with infiltrating signet ring cells involving the entire thickness of the gastric wall. The patient underwent a total gastrectomy associated with chemoradiotherapy.
Keywords: Radiation enteritis; Bowel obstruction; Malabsorption; Treatment
La enteritis por radiación es una patología causada por la radiación que se suministra durante el manejo de neoplasias radiosensibles. Esta enfermedad puede clasificarse en enteritis aguda o crónica, en las cuales es posible que se desarrollen síntomas por malabsorción u obstrucción intestinal, que alteran la calidad de vida de los pacientes. Presentamos el reporte de caso de un paciente masculino de 67 años, con antecedente de adenocarcinoma difuso con células en anillo de sello infiltrante y compromiso de todo el espesor de la pared gástrica, quien había recibido un manejo quirúrgico mediante gastrectomía total, asociado a quimio-radioterapia. El individuo consultó por síntomas de obstrucción intestinal. En principio, se consideró la existencia de una recaída tumoral. Sin embargo, se corroboró que los síntomas estaban relacionados con el compromiso causado por la radiación.
Palabras clave: Enteritis por radiación; obstrucción intestinal; malabsorción; tratamiento
Introduction
Radiation enteritis is a side effect caused by radiation therapy. Two types have been described: Acute and Chronic Radiation Enteritis 1. Acute symptoms (abdominal pain, loss of appetite, diarrhea) occur within the first few hours or days after radiation therapy and can be managed with medical treatment 2-6.
Meanwhile, chronic radiation enteritis leads to malabsorption or chronic obstruction syndrome at least two months after the end of radiation therapy 7-10. It involves exclusion diagnosis, confirmed by a histopathological study, and it is characterized by the presence of fibrosis associated with obliterating arterial lesions 11-16. Its treatment is initially medical and, in the face of obstructive symptoms due to stenosis or fistulas, surgical resection of the digestive tract elements involved and ileocolic anastomosis in healthy areas is indicate 17-19.
Clinical case
67-year-old male who, in 2014, was diagnosed with diffuse gastric adenocarcinoma with cells in the infiltrating seal ring and involvement of the entire thickness of the gastric wall. The patient had received surgical management through total gastrectomy, together with neoadjuvant chemo-radiation therapy.
The patient was admitted to the emergency room on January 12, 2019 due to experiencing the following symptoms for 20 days: dysphagia to solids and liquids -gradually occurring- associated with a sensation of chest tightness during ingestion, as well as multiple food content emetic episodes and a 6 kg weight loss.
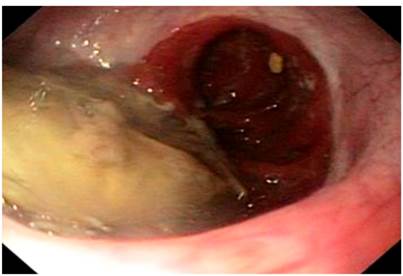
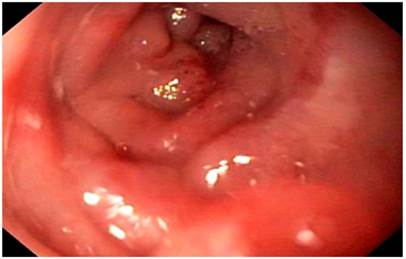
On physical examination, signs of dehydration were observed, with no other positive findings. In view of the history and symptoms described, an endoscopy of the upper digestive tract was performed. Food impaction was observed at the level of esophagojejunal anastomosis (Figure 1). A foreign body was removed, and an anastomosis was found, with no signs of tumor relapse. However, 10 cm from the anastomosis, a stenosis zone of 40% of the lumen was found, with a concentric decrease of the lumen due to a circumferential edema of the mucosa (Figure 2), which allowed the equipment to get in with a slight resistance, and biopsy samples were taken from the area.
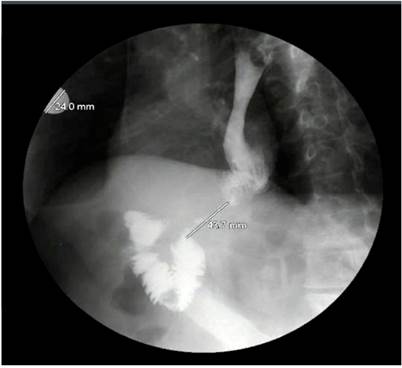
As a result of these findings, an x-ray of the digestive tract was performed (Figure 3), in which ileum stenosis of 43 mm, distal to anastomosis, was observed, suggesting the possibility of tumor relapse. Therefore, a computed tomography (CT) scan of the abdomen was performed, in which post-surgical changes of the total gastrectomy, Roux Y reconstruction, and thickening of the intestinal wall at sites of esophagojejunal and jejuno-jejunal anastomosis, with no signs of intestinal obstruction, were observed.
The patient was evaluated by the gastrointestinal surgery service, where it was considered he had a stenosis of the afferent loop, probably associated with a tumor relapse. Therefore, a liquefied diet was ordered, which was well tolerated by the patient. As a favorable evolution was observed, he was discharged, and outpatient follow-up was ordered to read the reports of the biopsy samples taken initially.
However, the patient was re-admitted to the emergency room 9 days after being discharge, as a result of the recurrence of low dysphagia for solids and liquids, and so he was hospitalized. The histopathology report described the presence of a jejunal mucosa with preserved architecture, an edematous and congestive lamina propria, as well as vascular congestion and increased mononuclear inflammatory infiltrate, accompanied by polymorphonuclear cells, which permeated the glandular crypts and the surface epithelium. No tumor injury or malignancy was identified. Thus, a mild chronic inflammation was considered.
Thus, a new endoscopy of the upper digestive tract was performed and a wide and permeable oesophagojejunal anastomosis was found, which allowed the equipment to reach the area without resistance. An inflammatory-looking mucosa was observed, and biopsy samples were taken from it. Likewise, the efferent loop was explored and an edematous and erythematous mucosa was observed, with a concentric decrease in the lumen due to a circumferential edema. Adequate visualization of the lumen was not achieved, but a stenosis of approximately 60% was found, which allowed the equipment to reach the area, but with some resistance. Therefore, it was not possible to move towards the healthy mucosa. Following this, the biopsy report informed that there were findings suggestive of malignancy and that congestive variations without signs of fibrosclerosis, granulomas or infectious changes were observed.
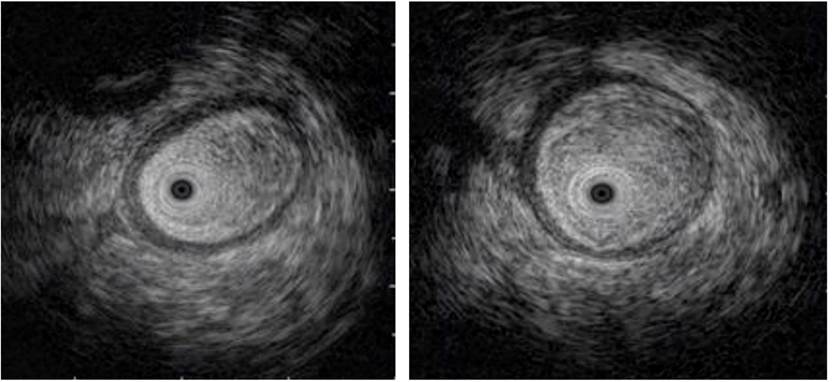
The study was complemented by an echo-endoscopy (Figure 4), in which the presence of edema of the loop wall was determined. Given these findings, radiation enteritis was highly suspected, and the patient was reassessed by the gastrointestinal surgery service, where it was considered that, in the event of non-improvement, he could benefit from surgical management. Therefore, a remodeling of the anastomosis was performed, and no tumor relapse was described in the histopathology report.
Discussion
Ionizing radiation is a common treatment in various types of neoplasms. However, gastrointestinal damage caused by radiation is a limiting factor of said therapy. Indeed, cancer survivors are known to have acute, and even chronic, symptoms after receiving radiation therapy. This reduces their quality of life and generates an additional cost to health systems.
In this context, the accurate diagnosis of damage by gastrointestinal radiation is a challenge, since, despite the increase in the recognition of damage and advances made regarding its molecular mechanisms, little is known about its pathophysiology and aggravating factors 1.
Advances on external radiation technique have been made since, when performing 3D simulation at a modulated intensity, toxicity is reduced. There is also an improvement in the definition of the target in relation to the tumor and the surrounding tissues, using combined rays of varying intensity. This allows for a better dose with less toxicity, given that the distribution of radiation over the volume of interest is more specific 2.
There are risk factors that can cause further damage to the gastrointestinal tract. This depends on the treatment provided to each patient. Radiation doses are the major determinants of the severity of acute damage and the late toxicity of normal tissue. Total dose, dose fractionation and treatment schemes have been found to play a key role regarding toxicity.
On the other hand, some additional risk factors include combined treatment modalities -such as surgery and concurrent chemotherapy-, history of abdominal surgery, and medical comorbidities (vascular diseases, connective tissue diseases, inflammatory bowel disease, and human immunodeficiency virus [HIV]) Either way, there are strategies to decrease the risk of gastrointestinal tract damage, such as hypofraction and reduction of radiation therapy doses 3,4.
Different mechanisms have been suggested to explain the sensitivity effect generated by combined therapies. These effects include changes in the kinetic cycle or synchronization in cell population replication. Some halopyrimidine drugs such as 5-fluorouracil, fluorodeoxyuridine, and iododeoxyuridine may sensitize tumors, inhibit effective DNA repair, and increase radiation-induced DNA damage 4,5.
Mechanisms of radiation damage
As a result of radiation, double-stranded DNA damage occurs in cells. This favors the onset of pathways leading to the activation of the tumor suppression gene P53, which results in a cycle stoppage, with repair of damage or apoptosis. Intestinal crypts are susceptible to this injury and cell death results in mucosal damage. It is important to consider that if apoptosis occurs in the stem cell, crypts cannot be regenerated, which contributes to the exposure of the lamina propria, which is normally sterile to germs 6.
On the other hand, there is vascular damage, which appears to be key in both acute and chronic radiation enteritis. It has been recognized that apoptosis of endothelial cells leads to stem cell dysfunction and subsequent stem cell damage 7.
All of this results in an inflammatory response mediated by T lymphocytes, macrophages, and neutrophils, damaging the extracellular matrix of the lamina propria and causing mucosal and submucosal involvement, which results in impaired immune recognition and bacterial translocation. In this way, the inflammatory process leading to fibrosis and stenosis is perpetuated 8.
Despite the cessation of the radiation therapy, the inflammatory process can continue, and its mechanisms are poorly understood. In fact, it is considered that there is an exaggerated response that contributes to severe ulceration, in which the chronic course of the disease is mediated by ischemia and fibrosis. In acute involvement, the main component is the damage of rapid cell proliferation, which results in reduced functional surface area and decreased secretion and motility capacity. Meanwhile, in the case of chronic involvement, obliterating endarteritis is the most important characteristic, with the consequent reduction of functional epithelial surface 9.
Secondary symptoms to radiation injury to the gastrointestinal tract
The gastrointestinal tract extends over a large surface, which is why it is easily affected by radiation. Symptoms can be acute and chronic, with clinical manifestations that may occur during or after radiation. Initially, acute mucosal damage and post-treatment inflammation occurs up to 90 days after radiation therapy. Such signs are usually reversible. Late symptoms may also appear months or years after radiation therapy. In fact, symptoms have been reported after 30 years of treatment, and they are usually related to transmural fibrosis and vascular sclerosis, whose reversibility likelihood is lower 10.
In the small intestine, the degree of damage depends on the dose and the segment volume included in the radiation field. Thus, the segment volume has a direct relationship with toxicity, regardless of the radiation dose used. Other predictors are concomitant use of chemotherapy and previous surgery. Similarly, radiation dose is related to late toxicity. In fact, the estimated toxicity in 5 and 50 % of patients with irradiation of 1/3 of the small bowel is 50 and 60 Gray (Gy), respectively. In addition, the estimated toxicity in 5 and 50% of patients with whole-bowel irradiation is 40 and 55 Gy 10,11.
When small bowel involvement occurs, early symptoms are nausea, vomiting, and abdominal pain during the first 2 weeks; there are related to the release of inflammatory cytokines. Diarrhea and abdominal pain occur during the first 2 weeks in 20-70% of patients undergoing abdominal or pelvic radiation therapy. These signs and symptoms are the result of direct damage to the mucosa, resulting in atrophy and decreased blood flow. It is important to consider that acute symptoms usually resolve within the first 3 weeks after radiation therapy is completed, due to regeneration of the epithelium 12.
In relation to late symptoms or chronic radiation enteritis, there are two main classes: Malabsorption syndrome with chronic diarrhea, and chronic pseudo-obstructive syndrome. Chronic diarrhea following radiation therapy is often secondary to different processes such as biliary acid malabsorption, bacterial overgrowth, fat malabsorption, rapid intestinal transit, and lactose intolerance. Stenosis, fistulas, and intestinal obstruction have been described as more severe late symptoms in 5-10% patients with severe damage and poor prognosis, with a 5-year mortality rate of 58%. Stenosis management is complex and results in unfavorable outcomes 12,13.
Short bowel syndrome, in which there is inadequate capacity for intestinal absorption, has also been described. The causes associated with this syndrome are surgery and intestinal fistula, which lead to malabsorption of macro and micronutrients, as well as of electrolytes and water. All this leads to rapid motility and intestinal transit, resulting in watery diarrhea and dehydration. This contributes to inadequate absorption of electrolytes, magnesium, and vitamin B12, and the occurrence of megaloblastic anemia, demyelinating neuropathy, and malabsorption of bile acids.
In short, malnutrition is secondary to short bowel syndrome, which, given the increase in mortality rates and the need for hospitalization, is an important finding related to chronic radiation enteritis. In addition, before radiation therapy begins, up to 33% of patients already have a baseline malnutrition condition, and after the procedure is performed, about 83% of them show significant weight loss.
Bacterial overgrowth, caused by the abnormal and excessive increase of bacteria in the small intestine, can also occur, which perpetuates malabsorption, weight loss, and malnutrition. This is also associated with stenosis, absence of ileocecal valve, and the colon 14.
Diagnostics
Diagnosis should be suspected according to clinical findings, which are characterized by nausea, vomiting, abdominal pain, diarrhea, or gastrointestinal bleeding, and are associated with a history of radiation therapy. The latter constitutes a diagnosis of exclusion.
Diagnosis is usually made from segmental involvement of the intestine, in regions of a radiation field observed in imaging and/or endoscopic studies, or histology reports. This diagnosis serves to exclude other causes and to establish the extent of the disease 15.
Thus, patients with abdominal pain, nausea, and vomiting could be initially assessed by performing an abdominal CT scan or magnetic resonance imaging, the findings of which include intestinal thickening, a mucosal superscan, and luminal stenosis. In the final stages of the disease, stenotic and obstruction segments of the small intestine occur as a result of fibrosis 15,16.
When endoscopic and histological studies are performed, findings compatible with radiation damage include pallor, friability, and telangiectasias, which can be multiple, large, and serpiginous. These changes tend to be continuous. Histological findings of radiation enteritis include diffuse collagen deposition, thickening of the mucosa and serosa, inflammatory cell infiltrates, vascular sclerosis, and occlusive vasculitis 16.
Treatment
In early stages of the radiation enteritis, pharmacological management may be provided to reduce intestinal transit. Loperamide and codeine phosphate act on the μ (ROM) receptors in the myenteric plexus to reduce symptoms of diarrhea. Correction of altered electrolytes and micronutrients such as potassium, magnesium, selenium, zinc and vitamin B12 levels is essential.
Regarding bacterial overgrowth, antimicrobial management using rifaximin is possible. Also, in case of malabsorption of bile acids, cholestyramine favors the reduction of episodes of diarrhea. Probiotics have been reported to decrease the severity of enteritis and a high concentration probiotic preparation of 8 live, lyophilized bacterial species is reported to reduce the incidence and severity of diarrhea.
This probiotic preparation consists of bacterial species that are normally found in human gastrointestinal microflora. These include 4 strains of lactobacilli (Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii, subspecie: bulgaricus), 3 strains of bifidobacteria (Bifidobacterium longum, Bifidobacterium breve and Bifidobacterium infantis), and 1 strain of Streptococcus salivary. Likewise, Lactobacillus rhamnosus reduces bowel movements and improves fecal consistency 17,18.
Diet modification is also important to reduce symptoms and the consumption of high-fiber and high-residue (raw vegetables) foods should be avoided. Hydration is important and using hypotonic fluids (500 mL/d) and saline glucose rehydration solution (100 mmol/L Na) is recommended 18.
On the other hand, parenteral nutrition is indicated in patients who cannot be managed with oral supplements. Indeed, it has been reported that patients with chronic radiation enteritis and managed with parenteral nutrition have a 5-year survival rate of 64%, which is associated with adverse effects due to vascular access and long-term liver dysfunction 18.
Surgery is indicated for chronic enteritis cases with signs of intestinal obstruction or in which fistulas, stenosis, perforation, and continuous bleeding occur. The objective of surgical management is to eliminate all radiation-induced lesions by resecting all involved elements and to avoid internal derivations. There is a significant risk of morbidity after surgery. In particular, the possibility of short bowel syndrome is high. In case of intestinal obstruction, surgery is controversial, reaching a morbidity of 30-50%, a mortality of 10-15%, and a 60% reoperation rate 19.
Conclusion
Radiation enteritis is an increasingly common, but at the same time, little known disorder. Its diagnosis is challenging for specialists. This disease causes malnutrition, dehydration, and diarrhea, and can be difficult to treat. Indeed, there are other associated conditions that can worsen these symptoms and increase confusion in diagnosis. Treatment options include strategies for managing associated conditions: Nutritional supplementation, pharmacological agents, surgery, and, in highly selected cases, small intestine transplantation. Additional studies are needed to determine the best approach to this condition, which is increasingly frequent and scarcely recognized.
REFERENCES
1. Letschert JG, Lebesque JV, de Boer RW, Hart AA, Bartelink H. Dose-volume correlation in radiation-related late small-bowel complications: a clinical study. Radiother Oncol. 1990;18(4):307-320. http://doi.org/10.1016/0167-8140(90)90111-9 [ Links ]
2. Meyer JJ, Willett CG, Czito BG. Emerging role of intensity-modulated radiation therapy in anorectal cancer. Expert Rev Anticancer Ther. 2008;8(4):585-593. http://doi.org/10.1586/14737140.8.4.585 [ Links ]
3. Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, Yarnold J, Horwich A. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353(9149):267-72. http://doi.org/10.1016/S0140-6736(98)05180-0 [ Links ]
4. Kasibhatla M, Clough RW, Montana GS, Oleson JR, Light K, Steffey BA, Jones EL. Predictors of severe gastrointestinal toxicity after external beam radiotherapy and interstitial brachytherapy for advanced or recurrent gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2006;65(2):398-403. http://doi.org/10.1016/j.ijrobp.2005.12.008 [ Links ]
5. Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620-5. http://doi.org/10.1200/JCO.2006.06.7629 [ Links ]
6. Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54(3):614-7. [ Links ]
7. Hauer-Jensen M. Late radiation injury of the small intestine. Clinical, pathophysiologic and radiobiologic aspects. A review. Acta Oncol. 1990;29(4):401-415. http://doi.org/10.3109/02841869009090022 [ Links ]
8. Rogler G, Gelbmann CM, Vogl D, Brunner M, Schölmerich J, Falk W, Andus T, Brand K. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol. 2001;36(4):389-98. http://doi.org/10.1080/003655201300051216 [ Links ]
9. Haydont V, Vozenin-Brotons MC. Maintenance of radiation-induced intestinal fibrosis: cellular and molecular features. World J Gastroenterol. 2007;13(19):2675-2683. http://doi.org/10.3748/wjg.v13.i19.2675 [ Links ]
10. Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol). 2007;19(10):790-799. http://doi.org/10.1016/j.clon.2007.08.011 [ Links ]
11. Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist?. Int J Radiat Oncol Biol Phys. 2005;62(5):1464-1471. http://doi.org/10.1016/j.ijrobp.2004.12.087 [ Links ]
12. Henriksson R, Bergström P, Franzén L, Lewin F, Wagenius G. Aspects on reducing gastrointestinal adverse effects associated with radiotherapy. Acta Oncol. 1999;38(2):159-164. http://doi.org/10.1080/028418699431564 [ Links ]
13. Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978-90. http://doi.org/10.3748/wjg.v16.i24.2978 [ Links ]
14. Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31(5):1213-1236. http://doi.org/10.1016/0360-3016(94)00419-L [ Links ]
15. Hussain A, Mahmood H, Thomas A, Frazer C, El-Hasani S. Does chronic radiation enteritis pose a diagnostic challenge? A report of three cases. Hong Kong Med J. 2008;14(4):327-330. [ Links ]
16. Kennedy GD, Heise CP. Radiation colitis and proctitis. Clin Colon Rectal Surg. 2007;20(1):64-72. http://doi.org/10.1055/s-2007-970202 [ Links ]
17. Yeoh EK, Horowitz M, Russo A, Muecke T, Robb T, Chatterton BE. Gastrointestinal function in chronic radiation enteritis--effects of loperamide-N-oxide. Gut. 1993;34(4):476-482. http://doi.org/10.1136/gut.34.4.476 [ Links ]
18. Nightingale J, Woodward JM; Small Bowel and Nutrition Committee of the British Society of Gastroenterology. Guidelines for management of patients with a short bowel. Gut. 2006;55 Suppl 4(Suppl 4):iv1-iv12. http://doi.org/10.1136/gut.2006.091108 [ Links ]
19. Regimbeau JM, Panis Y, Gouzi JL, Fagniez PL; French University Association for Surgical Research. Operative and long term results after surgery for chronic radiation enteritis. Am J Surg. 2001;182(3):237-242. http://doi.org/10.1016/s0002-9610(01)00705-x [ Links ]
Citation: Vargas-Rubio RD, Ovalle-Hernández AF. Radiation enteritis. Case report and literature review. Rev Colomb Gastroenterol. 2020;35(3):362-368. https://doi.org/10.22516/25007440.502
Received: January 25, 2020; Accepted: June 17, 2020











 text in
text in