Introduction
Aphids are r-strategist (Gadgil & Solbrig, 1972) insects characterized by their capacity for extremely rapid population growth together with a transient relationship with the host plant (Powell et al., 2006). Aphid colonies typically decline after an initial period of rapid growth. This last phase is largely due to the production of winged morphs and is stimulated by crowding, the presence of natural enemies, and the decrease in plant quality (Muller et al., 2001; Irwin et al., 2007). The curve of infestation for the same aphid species can be dramatically affected by the host plant despite keeping all the other factors equal (Larocca et al., 2011).
Macrosiphum euphorbiae (Thomas, 1878) (Hemiptera: Aphididae) is an important aphid pest that causes the most significant direct damage among the aphid species that attack tomato (Solanum lycopersicum L. 1753) (Perring et al., 2018). In a previous study concerning the development of M. euphorbiae colonies on tomato plants, we observed that cultivars and water stress affect the peak but do not interfere with the length of the infestation (Rivelli et al., 2013). This result was confirmed by field observations (Colella et al, 2014).
Several models of aphid population dynamics, which exclude predator inflicted mortality as a regulating factor, identify the initial number of aphids as the main determinant of the width of the density curve and/or size and the timing of the peak of maximum density (Kindlmann et al., 2007). In addition, some of these models take into account variations in the "aphid-carrying capacity" due to changes in host plant quality over time. Plant quality may largely depend on its phenology, which modifies the physiological priorities for resource allocation (Boege & Marquis, 2005).
In this study, we hypothesized that the initial number of aphids and/or the age of the plant are significant factors that can influence the duration and, consequently, the harmfulness of M. euphorbiae infestations in tomato.
Materials and methods
Tomato plants of the cultivar Rio Grande (pear-shaped processing tomato for paste and concentrated juice) were used for both M. euphorbiae rearing and the experiment. We bought tomato plants in polystyrene trays from a nursery that used seeds produced by OLTER (Piacenza, Italy). Seedlings were transplanted and grown individually in plastic pots (18 cm diameter, 19 cm height) with commercial soil (COMPO SANA® universal potting soil, International Kingenta Group, Italy).
Macrosiphum euphorbiae individuals were originally collected in a tomato crop in Scafati (Salerno, Italy) and reared on tomato plants at 20 ± 1°C, 65 ± 5% relative humidity, and a photoperiod of 18 h light:6 h dark.
The experiment was carried out at the University of Basilicata, Italy (40°36' N, 15°48' E), in the summertime, in a naturally lit and temperature-controlled greenhouse maintained at 20°C (with an oscillation between 15°C at night and 28°C at 12-14 pm). We compared three initial levels of infestation and two phenological phases of the plant. Nine plants were infested 7 d after transplant (pre-flowering stage; 18.0 ± 2.6 cm height; root biomass 0.10 ± 0.04 g; leaf biomass 0.43 ± 0.08 g) and 9 plants were infested 26 d after transplant (beginning of flowering; 39.5 ± 4.6 cm height; root biomass 1.5 ± 0.3 g; leaf biomass 6.4 ± 3.0 g). Three levels of initial infestation were obtained by placing 6, 10, and 15 M. euphorbiae adults (three replicates for each level of initial infestation: R1, R2, and R3), respectively, on plants. We selected this range of initial infestation to simulate a variable but incipient attack of the pest. We used apterous females for simplicity, even if it is more plausible that the infestation starts with winged females under natural conditions.
All plants were infested on the same day. The plants in the flower transition stage had been transplanted 19 d before the plants in pre-flowering. Plants were placed in the greenhouse in a completely randomized experimental design and regularly checked. The number of aphids in the whole plant was counted using a magnifying glass.
The infestation curves for each plant stage and each level of initial infestation were adjusted to second degree polynomials passing through the origin according to a "cumulative density model" (Kindlmann et al., 2007). In the present study, we used the polynomial parameters to calculate the axes of the vertex of the second-degree polynomials: the theoretical values of maximum abundance of aphids (the Y axis) and of the day needed to reach the maximum abundance (the X axis). The theoretical values of the maximum number of aphids and of the days needed to reach it were then analyzed with factorial ANOVAs, with "stage" (7 and 26 d after transplant, i.e., pre-flowering and flowering stages) and the initial level of infestation as fixed effects. The same analyses were also performed on the observed values of the maximum number of aphids and of the days needed to reach it. All the analyses in this study were performed using the R 3.2.3 software (R Core Team, 2014).
Results and discussion
The response curves for the three replicates of the pre-flowering and flowering stages are shown separately for each level of initial infestation in Figure 1. In all cases, values of adjusted R 2 are significant and the adjustments to second degree polynomial provide plausible values for the vertex. Positions of the theoretical and observed vertices of all the curves (mean values of three replicates) with the respective standard errors are shown in Figure 2. The mean value of the peak and time when the peak occurred for each plant stage are shown in Tables 1 (theoretical values) and 2 (observed values).
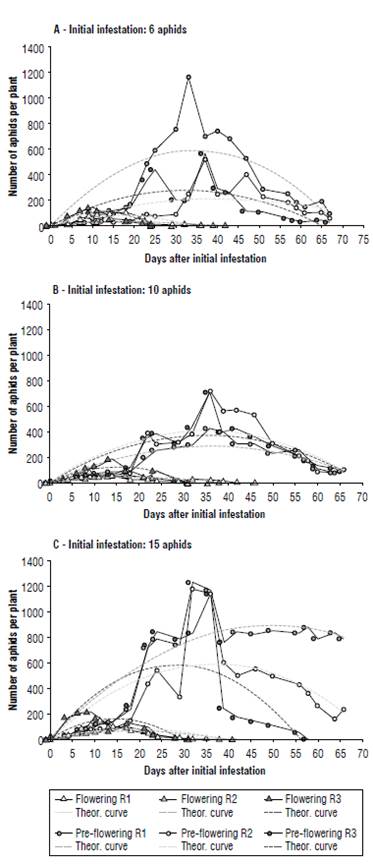
FIGURE 1 Curves of Macrosiphum euphorbiae infestation estimated for plants initially infested at the pre-flowering stage or at the beginning of flowering. A) Initial infestation: 6 aphids; B) initial infestation: 10 aphids; C) initial infestation: 15 aphids. Continuous lines connect the experimental points; dotted lines (theor.) represent the theoretical adjustments to second degree polynomial; R1, R2 and R3 are the three replicates for each level of initial infestation and stage.
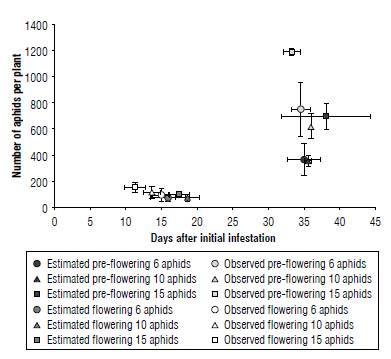
FIGURE 2 Maximum abundance of Macrosiphum euphorbiae colonies according to the initial infestation level (6, 10, or 15 aphids per plant) and the stage of the attacked plant (pre-flowering and flowering stages).
TABLE 1 Theoretical maximum abundance and time of maximum abundance according to the plant stage at the beginning of infestation and the initial number of aphids (mean ± standard error).
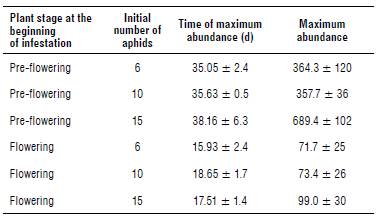
TABLE 2 Observed maximum abundance and time of maximum abundance according to the plant stage at the beginning of the infestation and the initial number of aphids (mean ± standard error).
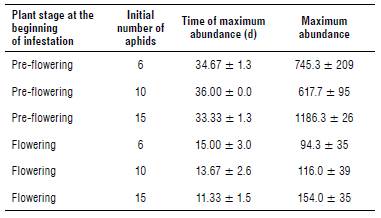
Aphid population reached a much higher peak on plants infested after 7 d from transplant (pre-flowering) compared to those subsequently infested (flowering) (theoretical values: F1,12 = 48.18, P<0.001; observed values: F1,12 = 83.28, P<0.001). Significant differences in the maximum abundance were also detected for the level of infestation (theoretical values: F2,12 = 4.45, P<0.05; observed values: F2,12 = 5.49, P<0.05) but not for the interaction between the phenological stage and the level of infestation. These differences are mainly due to the peak reached on plants initially infested with 15 aphids on the 7th d (Figs. 1C and 2). The vertices of the other curves are all very close.
The position of the peaks of infestation over time (the day after infestation when the aphid population reached its maximum abundance) was significantly affected only by the plant stage at the start of infestation (theoretical values: F1,12 = 57.35, P<0.001; observed values: F1,12 = 191, P<0.001).
Our results show that the duration of M. euphorbiae infestation in tomato mainly depends on the phenological stage of the host plant (it is higher and persists longer on early infested plants), while the degree of infestation seems to be irrelevant considering the initial levels used in this experiment.
Plant defense significantly changes with plant development from the seedling to juvenile to mature and senescent stages (Barton & Boege, 2017). Ontogenetic changes in plant defenses observed in many species do not only concern the intensity of the defensive response but also the mechanisms involved. This can be partially explained by the fact that allocation costs and resource allocation priorities vary as plants develop (Boege & Marquis, 2005).
Two important changes that can impact the development of insect infestations occur during the flower transition: the negative regulation of herbivory-induced jasmonic acid (JA) signaling (Gaquerel & Stitz, 2017) and the increase of the C:N ratio in the phloem sap (Corbesier et al., 2002). The repression of the JA signaling-based induction explains why the ability of tomato plants to induce defenses against Manduca sexta (Linnaeus, 1763) is lost when the reproductive stage is reached (Wolfson & Murdock,1990). The down-regulation of the JA signaling pathway mainly favors chewing insects. On the other hand, the increase of the C:N ratio in the phloem sap is unfavorable for aphids since amino acid availability significantly affects their growth and reproduction (Ponder et al., 2000; Karley et al., 2002). In the case of aphids, the nutritional quality of the host plant can have an even higher impact than that of the induced defenses (Battaglia et al., 2013).
In addition to the nutritional aspects, we must consider that plants have evolved multiple defense strategies against aphids, including constitutive as well as inducible factors (Nalam et al., 2019) that may change during ontogenesis. For example, expanded leaves have greater trichome density and resistance in tomato plants in reproductive stages than in vegetative ones (Mymko & Avila-Sakar, 2019). Furthermore, herbaceous plants usually show a significant increase in secondary chemistry across the entire ontogenetic trajectory (Barton & Koricheva, 2010).
The ontogenetic changes in the nutritional quality of the plant and in the defense strategies may explain the greater development of aphid colonies we observed when we infested tomato plants in the pre-flowering stage compared to the flowering stage. Our results confirm the better performance of aphids on young plants previously reported for other aphid-plant systems, as in the case of Diuraphis noxia (Kurdjumov 1913) on barley (Ma & Bechinski, 2009), and Myzus persicae (Sulzer, 1776) and M. euphorbiae on potato (Karley et al., 2002).
The best performance of aphids on young plants has practical consequences on the development of aphid infestations in the field and, therefore, on the control strategies that can be implemented. In fact, the extent of aphid infestations not only depends on the number of winged forms that move from the winter host to the herbaceous crops but also, to a large extent, on the phenological stage in which crop plants are at the time of the aphid invasion. The anticipation of the sowing time, when possible, allows plants to reach a less suitable phenological stage before aphid colonization takes place. This could be a strategy for the control of aphids, as several field studies also suggest (Perring et al., 1988; John et al., 2017; Alam et al., 2020).














