Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad de Odontología Universidad de Antioquia
versão impressa ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.25 no.1 Medellín jul./dez. 2013
ORIGINAL ARTICLES DERIVED FROM RESEARCH
THE EFFECT OF HANK'S BALANCED SALT SOLUTION (HBSS) STORAGE TIME ON TRANSDENTINAL DIFFUSION IN EXTRACTED THIRD MOLARS1
Francisco Araya2; Claudia Sommariva3; Gustavo Moncada4; Álvaro Cartagena,5; Claudia Letelier5; Osmir Oliveira Junior6; Javier Martín7; Eduardo Fernández7
1 This study makes part of PRI-ODO 0304/2012 UCHILE. It was partially financed by a Colgate-Palmolive-Chile Grant. The authors declare having no conflict of interest
2 Dental Surgeon, Universidad de Chile
3 Assistant Professor, Department of Restorative Dentistry, Universidad de Chile
4 Professor, Department of Restorative Dentistry, Universidad de Chile
5 Instructors, Department of Restorative Dentistry, Universidad de Chile
6 Professor, Department of Restorative Dentistry, Cosmetic Dentistry Studies, Universidade Estadual Paulista, São Paulo, Brasil
7 Assistant Professor, Department of Restorative Dentistry, Universidad de Chile. E-mail Address: edofdez@yahoo.com
SUMBITTED: MARCH 5/2012-ACCEPTED: OCTOBER 15/2013
Araya F, Sommariva C, Moncada G, Cartagena A, Letelier C, Oliveira O Jr et al. The effect of Hank's Balanced Salt Solution (HBSS) storage time on transdentinal diffusion in extracted third molars. Rev Fac Odontol Univ Antioq 2013; 25(1): 158-175.
ABSTRACT
INTRODUCTION: the solution used to store dentin disks while being studied has proven to be vital in reproducing possible in vivo conditions, and it is ultimately critical in evaluating hydraulic conductance studies. The goal of this in vitro study was to determine transdentin filtration rate variation in human dentin disks after 2, 4, 6, 7, 14, 21, and 28 days stored in Hank's Balanced Salt Solution (HBSS), 10% formalin, and saline solution. METHODS: this study included 41 unerupted healthy human third molars. These teeth were disinfected in 0.1% thymol for 24 hours before being embedded in epoxy resin blocks and then cut to obtain 1 mm thick dentin disks. The disks' hydraulic conductance was later measured, and then they were separated and stored in different solutions. They were sorted out in three groups: a) 10% formalin, b) Hank solution (HBSS) and c) saline solution. Hydraulic conductance was measured after 1, 2, 4, 6, 7, 14, 21, and 28 days. Statistical analysis was performed by ANOVA and Tukey's post hoc (SPSS v.15). RESULTS: the results suggest that the average of dentine disks' hydraulic conductance increased in all the storage solutions when comparing the analysis between day 1 and day 28. CONCLUSIONS: no significant differences were found (p > 0.005) in terms of hydraulic conductance among the various times the disks were stored in Hank's solution..
Key words: dentin, conductance, diffusion.
INTRODUCTION
Dentin forms the central structure of teeth. It is crossed by tubules that extend from the pulp to the dentino-enamel junction. These tubules provide the structure with physical characteristics such as permeability.1
The first description of substance permeation through dentine was made by Fritsch in 1914. In the 40s, Lefkowitz observed that a dye injected into a tooth's pulp penetrated the entire dentin in about half an hour. Some years later, Bodecker and Lefkowitz noted that a filler's dye applied on deep cavities was able to move to adjacent enamel areas as well as to dentin and pulp. Dentin's hydraulic conductance is defined as the ability of a fluid to pass through it,2 and the term dentin permeability refers to the passage of fluids, ions, molecules, particles, and bacteria into and through the dentin under different conditions.1, 3
Pashley et al have studied the relationship between dentin permeability and its topography and morphology under different solutions.1, 3-14 The permeability of various dental material components has also been studied, as well as their possible cytotoxic effect on pulp cells.5-20 Another field of research derived from dentin permeability is adhesive systems testing.15 Due to the humidity and complex nature of this tissue, adhesion to dentin and dentinal surface sealing remain a problem despite recent advances in adhesive dentistry. Several permeability models have been used to calculate the capacity of adhesive systems to seal dentin and to determine dentin adhesive strength of various adhesive systems, cements, and other dental materials.
Despite being a widely studied field, there is little information about dentin permeability at different maturation stages or about systemic variables that may affect maturation, composition, or permeability.
The existing information about how experimental models work to determine dentin permeability in vitro is scarce.
Among the variables included in dentin permeability in vitro studies, the solution in which dentin disks are stored while being studied has proven to be vital to reproduce possible in vivo conditions, and it is ultimately critical in evaluating hydraulic conductance studies. This happens because the solution in which samples are immersed determine the way disks' properties will be maintained, possibly influencing their permeability.21 The present study seeks therefore22 to analyze different storage solutions, presenting Hank's solution (Hank's Balanced Salt Solution, HBSS) as a new alternative.22
The use of this solution in dental trauma currently enjoys good recognition since it provides greater periodontal cell viability in case of avulsion. Also, its pH, osmotic concentration, and ionic components make of it an ideal material for storing dental tissue. 21, 23-27 It will be necessary to determine whether this substance is able to keep disks in optimal conditions to measure their hydraulic conductance, and therefore become a field for future research. Serum has proven to be an inadequate storage agent due to the large structural changes it causes in dentin, resulting in an excessive increase in permeability.28 In fact, both hardness and Young's modulus of elasticity decrease when samples are stored in serum, probably due to the loss of surface calcium, leading to dentin collagen exposure, which may affect the hydrolysis of exposed collagen fibers.
In endodontics, several solutions with different physiological characteristics have been used, especially for problems related to dentoalveolar trauma, such as dental avulsion. Tooth avulsion is one of the most severe forms of dental trauma, and it has been described as complete displacement of teeth from alveoli.
Given the complexity of this situation, the neurovascular system is highly affected, often resulting in loss of pulp vitality.29 As a result, for years endodontists have sought methods to keep teeth stable after avulsion for subsequent replantation.
This search resulted in Hank's Solution or HBSS (Hank's Balanced Salt Solution). This solution is slightly basic, with a pH ranging between 7.2 and 8.0. It is highly concentrated in Ca2 +, Mg2 +, Na+, PO34, and Cl+ ions, and its composition is comparable to dental mineral phases.21 Also, it has a low chemical potential to dissolve calcium phosphate phases in teeth, so it prevents surface demineralization. 23 Similarly, Hank's solution's osmolarity is approximately 300 mOm/l,24, 25 and since the optimum osmolarity for cell preservation ranges between 230 and 400 mOm/l, it is an ideal solution for this type of tasks.26, 27 In fact, multiple studies have proven the effectiveness of HBSS as a preservative of periodontal ligament cells in case of trauma, including the study by Ashkenazi, who showed that HBSS solution is the most effective storage agent for preserving viability, motility, and the clonogenic capacity of periodontal ligament cells after being stored for 24 hours at 22° C.30
Given the tissue conservation characteristics of Hank's solution, it has been thoroughly studied and its practical application is on the rise. It proved to be, for instance, an excellent suspension for dentin and enamel preservation in short-term studies on tooth mechanical resistance.23 However, it is important to note that Hank's solution turns out to be more expensive than other solutions, besides being unstable when exposed to light for short periods of time;,24 this is why the study of its characteristics, benefits, and contraindications is still in progress. The purpose of this in vitro study is to determine transdentin filtration variation rate (hydraulic conductance) in human dentin disks after 2, 4, 6, 7, 14, 21, and 28 days stored in HBSS solution, 10% formalin, and saline solution.
METHODS
In this observational study, we used 41 unerupted healthy human third molars extracted from subjects between 16 and 30 years of age, with a coronal diameter no greater than 12 mm. These teeth were obtained once patients signed an informed consent, and the study was approved by the Ethics Committee of Universidad de Chile School of Dentistry. After extraction, teeth were manually cleaned with a Gracey 13-14 curette (HuFriedy -USA) and disinfected for 24 hours in a 0.1% thymol solution (SIGMA, England); finally, they were stored in an HBSS solution at room temperature for two weeks or less.
The samples were later embedded in Bosh epoxy resin blocks of 25 mm in size. For this purpose, the molars were cleansed with distilled water and applied 37% orthophosphoric acid (Coltene-Whaledent) for 30 seconds. They were washed, dried, and brushed with cyanoacrylate, and then inserted in a cylindrical Vaseline silicone mold of 25 mm in diameter, using pink wax on their occlusal side to fix them. Finally, the epoxy resin was poured into the mold and left to polymerize for at least 48 hours.
Once the epoxy resin polymerized, sections were made perpendicularly to the larger axis of each tooth at the level of the crown, thus achieving disks 1 mm thick. These sections were made with an ISOMET BUEHLER LER (Lakebuff IL, USA) 1000 trimmer at 700 rpm, with 500 grams of pressure and under abundant cooling. Once the disks were obtained, the occlusal and pulp sides were regularized with abrasive paper (No. 600 silicon carbide) under running water in order to standardize thickness and to achieve surface smoothing. Each surface was etched in order to remove smear layers by using 37% ortophosphoric acid for 15 s on each surface, and then the samples were generously washed with water.
The trimmer was set at 700 rpm, with 500 grams of pressure on its cutting arm and under abundant cool water. The experimental model we used to measure flow was a modification to Pashley's equipment31, 32 and it was provided with a reservoir of distilled water in a vertical water column of 200 mm, connected to a stopcock. Then a silicone tube was used to make a connection with a graduated capillary tube horizontally positioned, to which a drop of air was added. The capillary tube was distally connected to a chamber where the disks under study were placed (dentin disks sealed by silicon rings) by means of a silicone tube (figure 1).
A modification to that used by Pashley; it has a vertical column of 200 mm of water, connected with silicone to a capillary tube, which in turn is connected to the chamber where dentin disks are placed.
Each disk under study was observed for 20 minutes, repeating each measurement three times a day to obtain a daily average. Furthermore, each disk was measured the day it was cut, and 2, 4, 6, 7, 14, 21, and 28 days afterwards.
Consequently, following this study's objectives, five experimental groups were formed, as follows: disks stored in Hank's solution (SIGMA, England) (n: 15); disks stored in 10% formalin (n: 18); disks stored in serum (n: 5); measurement with no disk (negative control), epoxy resin disk (positive control).
The permeation flow rate was measured by recording the initial position of the air bubble inside the capillary in order to determine its final position after 20 minutes(figure 2).
Measurement of the variation experienced by the air bubble inside the capillary during 20 minutes, in order to know the amount of water passing through the dentin.
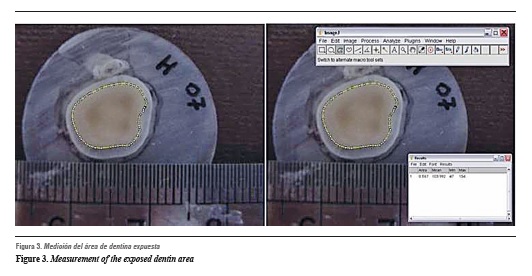
The measurements of each disk's exposed dentin area were calculated by the Image J computer software3333 (figure 3). After measuring each disk's area and flow rate, hydraulic conductance was calculated by the following hypothesis: hydraulic conductance corresponds to a formula that determines permeability, in this case dentin disks. Variable F corresponds to the flow rate of each experimental group; variable A is the dentine area exposed to the fluid; variable P is the intrapulpal pressure, which value corresponds to the height of the column with distilled water (200 mm), and variable t is time in minutes.
Once the dentin disk image is obtained with Image J, the required area is selected and the requested values obtained.
Statistical analysis was performed by two-way ANOVA test and Tuckey's post hoc (SPSS v.15). All this analysis was conducted on G* Power 3.1 software, and the sample was determined by p = 0.05, a statistical power of 0.8 and an effect size of 0.5.
RESULTS
From a total of 41 third molars in perfect conditions collected for this study, 41 dentin disks of 1 mm(± 0,01 mm), were obtained and divided into three groups, corresponding to the three storage solutions under study. The groups were:
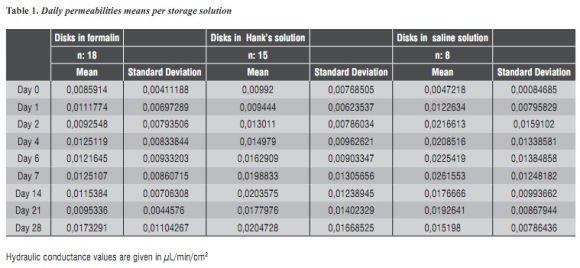
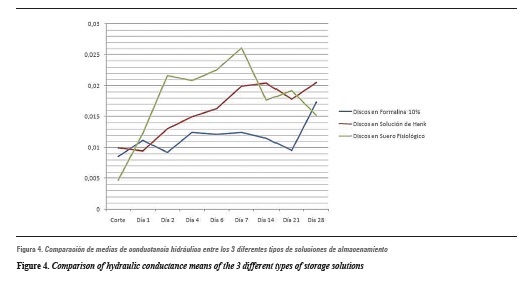
Table 1 shows the means and their respective standard deviations of the different permeability rates obtained in various storage media. It should be noted that n values in each solution were different, since in 10% formalin n = 18 samples, in Hank's solution n = 15 samples, and in saline solution n = 8 samples. Figure 4 shows the comparison of daily averages of the 3 study groups.
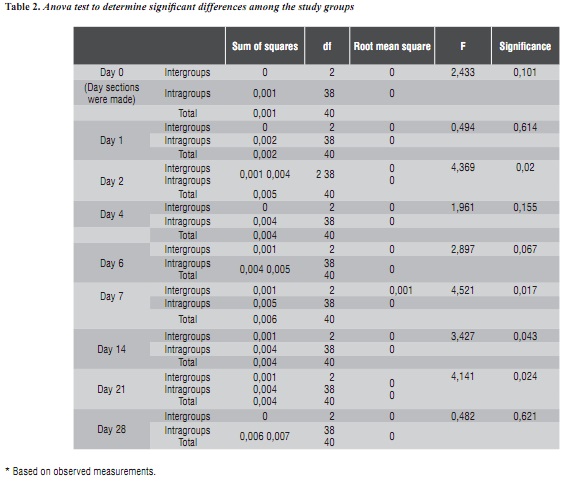
The groups do not show differences on days 0 (p = 0.101), 1 (p = 0.614), 4 (p = 0.155), 6 (p = 0.67) or 28 (p = 0.621). However, they do differ on days 2 (p = 0.020), 7 (p = 0.017), 14 (p = 0.043) and 21 (p = 0.024) (table 2).
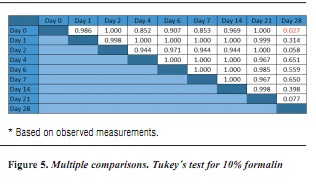
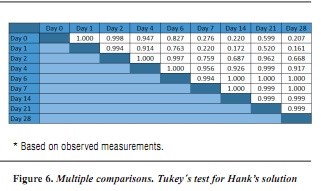
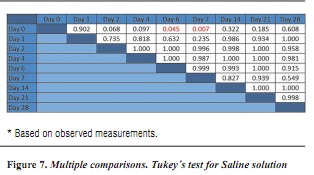
In order to determine which groups present significant difference, the post-hoc test is conducted (figures 5, 6 y 7).
The results show significant difference (p < 0.05) between formalin and saline solution on days 2 (p = 0.014) and 7 (p = 0.018) as well as between formalin and Hank's solution on day 14 (p = 0.038). The Tukey's post hoc test was conducted for each solution in order to identify the days in which significant differences occurred.
Based on the results, it is possible to observe significant differences (p < 0.05) in samples stored in formalin between days 0 and 28 (p = 0.027) and in samples stored in saline, solution between days 0 and 6 (p = 0.045) and 0 and 7 (p = 0.007).
DISCUSSION
This study arises from the need for materials to develop short- and long-term studies on dentin permeability without altering the agents by reactions among dental tissues, materials used, and storage solution. The alternatives currently tested have not proven to provide all of these conditions, since some agents such as saline solutions and other similar solutions have failed to demonstrate their usefulness as storage agents in the mid-term and others, such as formalin, have shown better results but they alter tissue characteristics that may eventually affect the results.21, 25, 29
The model used for this study is based on that used by the research group led by Pashley31 and other researchers afterwards. This model offers a simple, reliable tool: the diffusion chamber that allows to visually measure dentin disks' hydraulic conductance and to determine dentin permeability. Basically, the design of the present study differs in the hydrostatic pressure used, namely 20 cm of H2O at 20° C. Based on the technique used, we may establish a comparison with Sekimoto, who used the same model but with a pressure of 15 psi of N2 on a 0.2% phosphate saline solution, equivalent to 1054.8 cm H2O at 4° C. 34 Since the process is slow, many studies tend to significantly increase pressure in order to obtain larger values. In this case, and because of possible future uses of this study, we decided to use a pressure close to physiological pulpal pressure which according to several authors ranges between 12 and 18 cm H2O.35
The fact that dentin flow tends to move from the pulp towards the tooth's external portion is due to the difference in pressure between the environment and the pulp; since the latter is higher, it generates an expulsive flow of fluid from the pulp.12 However, this study was performed by producing flow from occlusal to pulpal, taking into account the results by Pashley, who pointed out that flow tends to decrease in pulpal-occlusal direction, due to an increased risk of tubule obliteration by pulpal products. Nevertheless, the variation found by this author between both flow directions was not statistically significant.36
In this study, orthophosphoric acid was used to treat the dentin surface because it tends to permeate obliterated surfaces increasing permeability in values ranging from 87 to 146%.37 Similarly, we attempted acid treatment under conditions and times similar to those used in clinical conditions, avoiding other type of etchers with limited clinical applications, such as citric acid.
Similarly, permeability rate was measured by means of distilled water instead of serum in order to avoid precipitation of salts, which may obliterate dentinal tubules.1, 28, 38
Hank's solution was used in this study with the intention of providing dentin permeability studies with a solution that has shown to maintain dentinal tissue over time, as it retains much of its mechanical, chemical, and even physiological properties. All this is due in large part to its similarities with tooth mineral phases, both in pH and osmolarity, besides sharing similar ions with them.
The results of this study allow us to infer some facts. Once the samples began to be analyzed, that is, immersed in storage solution and measured their permeability over time, their hydraulic conductance increased as days went bysimilar to what has been reported in the literature. Similarly, and considering figure 4, the group that showed the highest increases, particularly during the first days of study, was that of the samples stored in saline solution. Their counterpart was the samples stored in 10% formalin, which, although presented increased permeability over time, were the ones that presented the least variation when compared to baseline. For Goodis et al,28 this is probably due to the fixative property of formalin, which retains the dentinal tubules structure, producing smaller hydraulic conductance changes.
These results show significant differences (p < 0.05) between the formalin and serum samples for days 2 (p =.014) and 7 (p = 0.018), and also between formalin and Hank's solution samples for day 14 (p = 0.038). Also, Tukey's post hoc test in each solution shows significant differences in samples stored in formalin between days 0 and 28 (p = 0.027), and for samples stored in saline solution between days 0 and 6 (p = 0.045) and 0 and 7 (p = 0.007).
We may conclude that the agents under study present significant differences among themselves in some moments of the analysis but they cannot be comparable, at least in terms of hydraulic conductance, to the fact of storing samples in any storage solution. Moreover, variations in the samples, both in saline solution and in 10% formalin, showed significant differences at times, being consistent with the literature concerning the difficulty of predicting the behavior of dentin permeability, even in agents considered to be good preservative solutions such as formalin. Regarding Hank's solution, no significant difference was observed among the samples during the days of study, therefore proving the null hypothesis of this study.
However, we suggest performing further studies on dentin permeability and Hank's solution's behavior as a storage agent by means of projects that include even longer times than the ones evaluated in the present study. It is also recommended to check any reaction of the material against Hank's solution before analyzing any material in this solution.
CONCLUSIONS
- There are statistically significant differences among the various types of dental sample storage for 4 of the 9 days when the samples' hydraulic conductance was studied (days 2, 7, 14, and 21).
- The samples stored in 10% formalin were the ones that showed the greatest hydraulic conductance variation.
- The samples stored in Hank's solution did not show significant differences in hydraulic conductance during the study days.
- The diffusion chamber used in this study, derived from Pashley's model, is a simple and reliable tool for the study of dentine diffusion.
REFERENCES
1. Reeder OW Jr, Walton RE, Livingston MJ, Pashley DH. Dentin permeability: determinants of hydraulic conductance. J Dent Res 1978; 57(2): 187-193. [ Links ]
2. Maita E, Simpson MD, Tao L, Pashley DH. Fluid and protein flux across the pulpodentine complex of the dog in vivo. Arch Oral Biol 1991; 36(2): 103-110. [ Links ]
3. Pashley DH, Andringa HJ, Derkson GD, Derkson ME, Kalathoor SR. Regional variability in the permeability of human dentine. Arch Oral Biol 1987; 32(7): 519-523. [ Links ]
4. Pashley DH, Nelson R, Williams EC. Dentin hydraulic conductance: changes produced by red blood cells. J Dent Res 1981; 60(10): 1797-1802. [ Links ]
5. Pashley DH, Michelich V, Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent 1981; 46(5): 531-537. [ Links ]
6. Pashley DH, Thompson SM, Stewart FP. Dentin permeability: effects of temperature on hydraulic conductance. J Dent Res 1983; 62(9): 956-959. [ Links ]
7. Derkson GD, Pashley DH, Derkson ME. Microleakage measurement of selected restorative materials: a new in vitro method. J Prosthet Dent 1986; 56(4): 435-440. [ Links ]
8. Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod 1986; 12(10): 465-474. [ Links ]
9. Pashley DH Depew DD. Effects of the smear layer, Copalite, and oxalate on microleakage. Oper Dent 1986; 11(3): 95-102. [ Links ]
10. Pashley EL, Tao L, Derkson G, Pashley DH. Dentin permeability and bond strengths after various surface treatments. Dent Mater 1989; 5(6): 375-378. [ Links ]
11. Pashley DH. Mechanisms of dentin sensitivity. Dent Clin North Am 1990; 34(3): 449-473. [ Links ]
12. Pashley DH. Dentine permeability and its role in the pathobiology of dentine sensitivity. Arch Oral Biol 1994; 39 Supl: 73S-80S. [ Links ]
13. Gillam DG, Mordan NJ, Newman HN. The Dentin Disc surface: a plausible model for dentin physiology and dentin sensitivity evaluation. Adv Dent Res 1997; 11(4): 487- 501. [ Links ]
14. Pashley DH, Livingston MJ, Reeder OW, Horner J. Effects of the degree of tubule occlusion on the permeability of human dentine in vitro. Arch Oral Biol 1978; 23(12): 1127-1133. [ Links ]
15. Lanza CR, de Souza Costa CA, Furlan M, Alécio A, Hebling J. Transdentinal diffusion and cytotoxicity of self-etching adhesive systems. Cell Biol Toxicol 2009; 25(6): 533-543. [ Links ]
16. Lessa FC, Nogueira I, Huck C, Hebling J, Costa CA. Transdentinal cytotoxic effects of different concentrations of chlorhexidine gel applied on acid-conditioned dentin substrate. J Biomed Mater Res B Appl Biomater 2010; 92(1): 40-47. [ Links ]
17. Lessa FC, Nogueira I, Vargas Fda S, Spolidorio DM, Hebling J, Garcia-Godoy F, Costa CA. Direct and transdentinal antibacterial activity of chlorhexidine. Am J Dent 2010; 23(5): 255-259. [ Links ]
18. Soares DG, Ribeiro AP, Sacono NT, Coldebella CR, Hebling J, Costa CA. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast- like MDPC-23 cells. Int Endod J 2011; 44(2): 116-125. [ Links ]
19. Turrioni AP, de Oliveira CF, Basso FG, Moriyama LT, Kurachi C, Hebling J et al. Correlation between light transmission and permeability of human dentin. Lasers Med Sci 2012; 27(1): 191-196. [ Links ]
20. Lima AF, Lessa FC, Mancini MN, Hebling J, Costa CA, Marchi GM. Transdentinal protective role of sodium ascorbate against the cytopathic effects of H2O2 released from bleaching agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109(4): e70-76. [ Links ]
21. Thomas T, Gopikrishna V, Kandaswamy D. Comparative evaluation of maintenance of cell viability of an experimental transport media "coconut water" with Hank's balanced salt solution and milk, for transportation of an avulsed tooth: An in vitro cell culture study. J Conserv Dent 2008; 11(1): 22-29. [ Links ]
22. Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent 2009; 20(1): 1-9. [ Links ]
23. Habelitz S, Marshall GW Jr, Balooch M, Marshall SJ. Nanoindentation and storage of teeth. J Biomech 2002; 35(7): 995-998. [ Links ]
24. Anjum A, Otsuki M, Matin K, Tagami J. Preservation in the liquid media produces alterations in enamel surface properties. J Dent 2009; 37(11): 884-890. [ Links ]
25. de Souza BD, Bortoluzzi EA, da Silveira Teixeira C, Felippe WT, Simões CM, Felippe MC. Effect of HBSS storage time on human periodontal ligament fibroblast viability. Dent Traumatol 2010; 26(6): 481-483. [ Links ]
26. Blomlöf L. Milk and saliva as possible storage media for traumatically exarticulated teeth. Swed Dent J Supl 1981; 8: 1-26. [ Links ]
27. Lindskog S, Blomlöf L, Hammarström L. Mitoses and microorganisms in the periodontal membrane after storage in milk or saliva. Scand J Dent Res 1983; 91(6): 465-472. [ Links ]
28. Goodis HE, Marshall GW Jr, White JM. The effects of storage after extraction of the teeth on human dentine permeability in vitro. Arch Oral Biol 1991; 36(8): 561-566. [ Links ]
29. Gopikrishna V, Baweja PS, Venkateshbabu N, Thomas T, Kandaswamy D. Comparison of coconut water, propolis, HBSS, and milk on PDL cell survival. J Endod 2008; 34(5): 587-589. [ Links ]
30. Ashkenazi M, Marouni M, Sarnat H. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in four at room temperature. Endod Dent Traumatol 2000; 16(2): 63-70. [ Links ]
31. Outhwaite WC, McKenzie DM, Pashley DH. A versatile split-chamber device for studying dentin permeability. J Dent Res 1974; 53(6): 1503. [ Links ]
32. Ozok AR, Wu MK, Wesselink PR. Comparison of the in vitro permeability of human dentine according to the dentinal region and the composition of the simulated dentinal fluid. J Dent 2002; 30(2-3): 107-111. [ Links ]
33. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7): 671-675. [ Links ]
34. Sekimoto T, Derkson GD, Richardson AS. Effect of cutting instruments on permeability and morphology of the dentin surface. Oper Dent 1999; 24(3): 130-136. [ Links ]
35. Ciucchi B, Bouillaguet S, Holz J, Pashley D. Dentinal fluid dynamics in human teeth, in vivo. J Endod 1995; 21(4): 191-194. [ Links ]
36. Greenhill JD, Pashley DH. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res 1981; 60(3): 686-698. [ Links ]
37. Tagami J, Tao L, Pashley DH, Hosoda H, Sano H. Effects of high-speed cutting on dentin permeability and bonding. Dent Mater 1991; 7(4): 234-239. [ Links ]
38. Camps J, Martin P, Ladeque P, Rieu R, Fuseri J. Influence of tooth cryopreservation on human dentin permeability, in vitro. Dent Mater 1994; 10(3): 210-214. [ Links ]











 texto em
texto em