INTRODUCTION
Candida is a commensal fungus present in the normal microbiota of the oral mucosa, which can have an opportunistic and pathogenic pattern in mammals, causing several forms of infection ranging from mild to severe. The latter constitute a systemic condition that compromises health, especially in individuals with a weakened immune system,1 like those suffering from noncommunicable diseases like cancer, and even more in those subjected to antineoplastic treatments, which are generally considered immunosuppressive factors, indirectly responsible for infections in the oral mucosa because of fungi like Candida.2
Cancer treatments are intended to increase and improve the potential survival of patients.1 They are also called antineoplastic therapies, including chemotherapy, radiotherapy, radical surgery, or a combination of these. They produce debulking and decrease in tumor size, causing systemic cytotoxic effects, which, depending on cancer stage, cause neutropenia and this in turn constitutes the main risk factor for serious lesions that indirectly affect the oral mucosa.1-3 Antineoplastic therapies, in their different management, application and dosage schemes, produce parallel direct and indirect oral complications, which can be reversible or irreversible and facilitate the proliferation of fungal microorganisms like Candida, with the consequent oral candidiasis (OC).4,5 This infection comes with factors described in various studies, such as aggravating adverse effects including hyposalivation and xerostomia, which favor colonization or infection due to the consequent decrease of pH in the oral environment, since Candida colonizes in acidic oral environments.6-8
Theoral cavity offers a conducive environment for the colonization of opportunistic microorganisms like Candida; however, this microorganism is not pathogenic in 100% of cases because normal bacterial microbiota and the immune system limit its growth and slow its excessive proliferation, thus maintaining a balance. 9-11 In patients under antineoplastic therapies, Candida infections increase their pathogenicity since they tend to occur faster than bacterial infections during neutropenia, representing 2-10% of the infections microbiologically confirmed in oncological neutropenic patients.12-17
Based on this evidence, the purpose of this article is to describe the pathogenic behavior of Candida in the oral mucosa of patients under antineoplastic therapies, detailing aspects such as nature and structure of the microorganism, the underlying infectious process, predisposing factors for oral candidiasis, virulence factors, pathogenesis, clinical presentation, and treatment.
CLASSIFICATION AND PREDISPOSING FACTORS
Yeasts of the Candida genus are present in soil, freshwater, vegetables, fruits, grains, and in general in any substance rich in simple carbohydrates. In addition, they are common inhabitants of the digestive and respiratory apparatuses and mucocutaneous areas in humans and domestic animals.2 The Candida genus includes nearly 154 species; the most commonly isolated in human beings are C. albicans, C. glabrata, tropicis, C. parapsilosis, C. dubliniensis, and C. krusei, which are often responsible for mucocutaneous infections, with C. albicans being the most relevant in terms of pathogenicity. 18 The human gastrointestinal system, including the mouth, has a small but constant population of C. albicans. Two factors have been described as regulators of the number of yeasts in the adult18
Intestinal flora: it exerts control over the population density of yeasts (mainly lactobacilli and anaerobic bacteria) by antimicrobial factors, adhesion inhibitors, oxide-reduction potentials, and competition for available nutrients.18-19
Diet: the excessive intake of fresh fruits, sweets, or other fermentable substances results in a considerable increase in the number of intestinal yeasts, particularly C. albicans, related to the pH decrease resulting from intestinal and oral fermentation.20
The normal skin can also present flora of resident yeasts, such as C. parapsilosis, C. guillermondii, and C. krusei. Other species, such as C. albicans and C. tropicalis, are not regularly found in normal skin, except in the anogenital region and around the mouth, as in the corners of the lips. C. albicans can also be isolated in the normal vaginal mucosa, and less frequently C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei.22-24 In optimal health conditions, colonization of the oral cavity by Candida and its species, especially C. albicans, does not cause disease and is not evident unless there are predisposing and etiologic factors for the development of the infection itself.24-29
The ability of Candida to colonize and take on a pathogenic behavior is related to the nature and degree of compromise of the individual’s immune system. Studies worldwide classify pathogenic Candida and OC in immunosuppressed patients30 as a result of various noncommunicable diseases, including cancer and its treatment, depending on the origin and predisposing factor, as of chronic origin or by debilitating, cytotoxic, iatrogenic and hematologic diseases (Table 1).31-36 Other predisposing and etiologic factors associated with the onset of pathogenic Candida and OC result from the destruction of salivary glandular acini caused by radiotherapy directly or close to the head and neck; this causes xerostomia or a feeling of dry mouth, favoring Candida colonization.25-36
The interest in oral infections caused by fungi of the Candida genus in immunosuppressed and/or medically compromised patients as a result of tumors has increased in recent years,23 resulting in a large amount of research focused on the identification of C. albicans as the main agent involved in OC. Some authors claim that OC is a superficial mycosis; however, depending on the host’s immune status and the location of the clinical manifestations, it may be superficial or deep.1-6
ORAL CANDIDIASIS IN CANCER PATIENTS
Infections, especially the fungal ones, are a common complication in cancer patients.37,38 They cause morbidity and mortality and a significant increase in disease costs. Several factors contribute to the increased risk of infection in these patients, such as defects in humoral and cellular immunity due to the basic oncologic pathology, side effects of the cytostatic or radiotherapeutic treatment, or those associated with oncological malnutrition and lesions in anatomical barriers (traumas).38
Regardless of the affected organ or system, cancer patients subjected to antineoplastic treatment, extensive surgeries, long hospitalizations with prolonged intravascular catheters and other devices that break the natural barriers, are easy targets of Candida colonization and infection not only in the mouth, but also in any part of the skin and other mucous membranes.39,40 Oral candidiasis in this type of immunocompromised patients usually spreads through the bloodstream or the upper gastrointestinal tract to other parts of the body and produce a severe infection called candidemia, significantly increasing morbidity and mortality38,41.
Studies reported by Puig-Asensio et al39 demonstrate the presence of C. albicans in the oral cavity as one of the most frequent species: in 45-65% of healthy children, 50-65% of denture patients, and 90% of leukemia patients; in the normal population, cases of asymptomatic Candida carriers have been reported in 17%. There are also other forms of candidiasis and candidemia in patients with tumors of hematological origin, in which the appearance of the fungus and infection is attributed to the state of oncological immunosuppression due to the cytotoxic action of chemotherapy on the oral environment; the presence of microorganisms other than fungi has also been reported.39
Authors like Verma et al42 and Morais et al43 report the relationship between the presence of Candida and gastrointestinal complications in patients with post- chemotherapy leukemia, as well as a high prevalence of OC in children as a common infection in patients with acute lymphocytic leukemia (ALL). The indirect relationship of OC to cancer is becoming increasingly evident, either due to antineoplastic treatment or as a condition inherent to the disease itself.
MICROSTRUCTURE, ETIO- PATHOGENESIS AND PATHOGENICITY FACTORS
The consulted literature reports that, microscopically, C. albicans does not show significant morphohistological differences under the studied oncologic conditions; however, the characteristics of this fungus in patients under antineoplastic therapies are controversial and scarcely reported in the literature. Authors like Ramla et al claim that chemotherapeutic agents, such as palladium and cisplatin complexes, can stimulate epithelial cellular respiration of the oral mucosa, causing major changes in the growth of the mycelium and inducing the formation of pseudohyphae more quickly.44 Ueta et al reported that the effects of antineoplastic drugs on oral epithelial cells enable C. albicans colonization and, biologically, an increase in the amount of proteinase, responsible for the destruction and/or inflammation of oral tissues affected by the fungus. The same authors suggest that, under these conditions of high virulence, this microorganism can persist in the oral cavity for a longer period than in a systemically healthy patient, and the clinical evolution of the infection also takes longer to be resolved. However, the effect of cancer treatment on the virulence of C. albicans is still under research.44,45
In a cancer patient, OC usually has microscopic characteristics similar to those of any other patient not oncologically affected (Figure 1), but it is interesting to note the reports on antifungal susceptibility against antifungal therapies and prophylaxis such as fluconazole, as some authors point out that there is a high resistance to pharmacological therapies under antineoplastic conditions.46
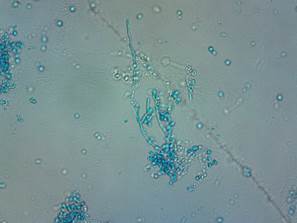
Figure 1 Microscopic structure of a pseudomycelium of C. albicans obtained from oral cavity, seen to 400X. Staining with lactophenol blue. Source: Universidad Metropolitana Microbiology Laboratory (Source: by the authors)
The pathogenesis of OC is complex and implies different factors and mechanisms of fungus and host.32,46 The possibility for Candida to colonize oral mucosa depends both on the effectiveness of the host’s defensive mechanisms and the capacity of the fungus to adhere, grow, and colonize.47 The balance between colonization and candidiasis is related to the ability of Candida to modulate the expression of virulence factors in response to environmental changes, combined with the competence of the host’s immune system and the antifungal therapy that can be implemented in the infected patient.9,10-15,32
In cancer patients under antineoplastic therapies, the pathogenic, virulent, and molecular changes are not as noticeable as in other immunological conditions, since the pathogenesis of OC is usually the same; however, Ramla et al conducted a study in a population subjected to chemotherapy for a prolonged period, and their C. albicans isolates showed that it produced greater amounts of phospholipase enzyme, increasing the relative risk of morbidity, suggesting the existence of a greater virulence. For Ramla et al, cancer patients are more colonized by Candida than healthy patients.44
Infection by Candida species is usually produced in the mycelium phase, stimulating the local secretion of numerous proinflammatory and immunoregulatory cytokines by epithelial cells.33 These cytokines stimulate the chemotaxis and innate immunity with local infiltration of macrophages, neutrophils, and T-lymphocytes, so their low levels provide high susceptibility to oral infections by Candida.44-47
The hypha is the invasive and pathogenic form of Candida. It causes the clinical manifestations of OC and has a specific protein called Hwp-1, which mediates the fungus adhesion to the keratinocyte of the epithelium.10 This protein forms covalent binding links to epithelial cells mediated by adhesins, which increase the adhesion by up to 80%. The deep penetration of the microorganism into the epithelium is mediated by the formation of the hypha, which uses thigmotropism as a guiding mechanism.48,49 (Figure 2).
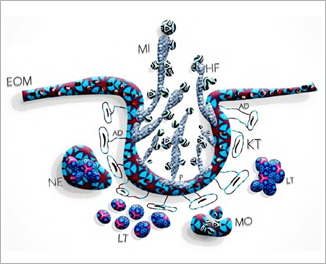
Figure 2 Pathogenesis of Candida infection in the oral cavity. EOM: epithelium of the oral mucosa, MI: mycelium, HF: hypha, AD: adhesins, KT: keratinocytes, NE: neutrophil, P: protein Hwp-1, LT: lymphocytes T, MO: macrophages (Source: by the authors)
To grow, Candida needs to obtain and metabolize nitrogen, and it has a transcriptional regulator containing zinc receptors, known as the GATA factor, which ensures the efficient use of the zinc sources available to the fungus. All this activates the expression of catabolic nitrogen pathways when the sources of zinc are absent or limited, a phenomenon known as repression.50 Authors like Pemán et al state that these repressive metabolic situations have been highly detected in patients in critical conditions of cancer, so it is considered an indicator of invasive candidiasis. Under normal conditions, this phenomenon causes fungal dimorphism, growth, and the ability to use serum amino acids to remain unchanged. This indicates the importance of nitrogen regulation in the virulence of C. albicans, which, besides being the most common pathogen fungus in humans, has an abundant repertoire of virulence mechanisms that favor colonization and infection in the host, in addition to other predisposing factors.50 (Table 2).
CLINICAL CHARACTERISTICS
In oncological immunosuppression circums- tances, the various Candida species can produce disease in any organ, especially in the oral cavity, the gastrointestinal system and the genital areas. Studies conducted by Paz et al reported the presence of OC in 57.14% (8/14) of cancer patients at the start of oncological treatment, and positive colonization in 61.5% (8/13), of which 46.2% corresponds to C. albicans and 15.4% to other species. During treatment and at the end of oncology therapy, 75% (9/12) of positive colonizations were identified, with 41.6% due to C. albicans and 33.3% to other species, such as C. krusei (30%), C. tropicalis (50%), and C. parapsilosis (10%).
C. albicans increased by 75% (9/12) at the end of oncologic therapy; the other species remained at the same frequency.51
C. albicans is responsible for most OCs, with different clinical manifestations, like superficial candidiasis, acute disseminated invasive candidiasis (IC), invasive candidiasis of a single organ (difficult to differentiate microbiologically and clinically from the disseminated form), chronic disseminated candidiasis (CDC), and candidiasis related to central venous catheter (CVC). Oral candidiasis is included in the superficial type but, depending on the severity of the effects of antineoplastic treatments, it can evolve to the more invasive, chronic, or disseminated form.38,44,51,52
The clinical onset of OC, in both patients under antineoplastic therapies and those systemically healthy, is usually symptomatic and produces burning in the oral cavity.
Sometimes the patient is unaware of the presence of the disease, which gradually results in the appearance of whitish and erythematous lesions, which cause difficulty in the intake of solids and, as the degree of affection on the oral mucosa increases, may also prevent fluid intake.1,4,8,53
Oral candidiasis has different clinical presentations. Singh and other authors8,54,55 classify it like this:
Pseudomembranous candidiasis (PSC): is the most prevalent (occurs in 70% of cases) and is more related to cancer and its treatment. It shows like a whitish pseudoplaque or pseudomembrane that can be removed with pressure or scraping, leaving an erythematous mucosa below.54-56
Chronic hyperplastic candidiasis (CHC): shows as a white hyperkeratosic spot, with or without epithelial tissue hyperplasia. It cannot be removed by scraping.54
Erythematous candidiasis (ES): shows intense reddish areas in the oral mucosa, often below a prosthesis (known as subprosthetic stomatitis) or after prolonged antibiotic therapy.54-57
Angular Cheilitis (AC): occurs less frequently than other types of OC and is rarely associated with oncological factors. It expresses with erythema, fissures, and scabs in the corners of the lips.4,55
The most common forms of OC reported in cancer patients in antineoplastic treatment are PSC (Figure 3) and ES, while CHC is rarely reported.56
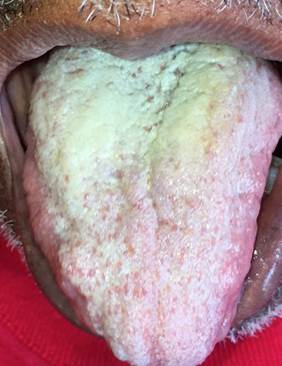
Figure 3 Clinical characteristic of an oncological patient in antineoplastic treatment with acute pseudomembranous candidiasis in dorsum of tongue, 4 months of evolution, asymptomatic and difficult to remove by scraping (Source: by the authors).
Regardless of its etiologic factor, OC is most frequently located in dorsal areas of the tongue, leading to a diffuse loss of the filiform papillae, which in turn leads to lingual atrophy and tends to manifest itself as a red plaque often accompanied by significant changes in taste, or dysgeusia.57 Lesions on the palate, edentulous alveolar ridges, gingiva, and floor of the mouth are less frequent. PSC may be accompanied with burning pain, sudden changes of taste while eating and halitosis when food is not consumed. AC is another clinical presentation of OC; it is often uncomfortable and can cause pain when opening the mouth. Therefore, the symptoms of OC can have a significant impact on quality of life and interaction with others, as it can affect nutrition and phonation.1,10,22,34,57
TREATMENT OF ORAL CANDIDIASIS
The therapies currently available for OC include a limited number of antifungal agents that have similar mechanisms of action. Most of these medicines act on the fungus’ cell membrane sterols or against the enzymes that regulate the synthesis of nucleic acids.57 The Candida fungus is similar to the eukaryotic cells of mammals; because of this, antifungal agents interfere with the metabolic pathways of human cells, so they have higher toxicity than many antibacterial drugs. In the OC of patients subjected to antineoplastic therapies, the initial pharmacological treatment should be topical; in severe or resistant cases, topical therapy should be combined with the systemic pathway.
There are three large groups of antifungal agents for the pharmacological treatment of OC: 1. Polyenes (Nystatin and Amphotericin), 2. Imidazoles (Clotrimazole, Miconazole, and Ketoconazole) and 3. Triazoles (Fluconazole).54-57
Nystatin is the most useful and effective antifungal polyene in the initial treatment of OC. It is usually prescribed in suspension, in doses ranging from 1 to 6 ml from 5 to 14 days, depending on the complexity of the infection.56-59
Authors like Castan et al describe a topical antifungal treatment, consisting of mouthwashes of sodium bicarbonate to alkalize the environment: 2.5 cc diluted in 50 cc of water four times a day for two minutes, after which the patient should not rinse the mouth cavity nor eat food for one hour. The application of 5 cc of miconazole topical gel three times a day is recommended as adjuvant therapy, massaging the infected areas for two minutes.59 Miconazole and Ketoconazole have the potential to interact with other medications, like hypoglycemics. Fluconazole and Itraconazole are newly introduced, water-soluble antifungals that are well excreted by the kidney without the hepatotoxicity of Ketoconazole.
Antifungal therapy should be accompanied with defocalization and hygiene of the oral cavity, as well as the use of Chlorhexidine mouthwashes. In addition, it is critical to provide recommendations in the use and disuse of dentures as a preventive measure for OC.57
CONCLUSIONS
Antineoplastic therapies have cytotoxic effects on the oral mucosa, which can be reversible or irreversible, direct or indirect. The mucous epithelial cells have a greater susceptibility to these effects due to their high degree of replacement or reepithelialization, resulting in symptomatic conditions in the oral cavity, which in turn become triggers and promoters of colonization by Candida.
Regardless of cancer location, antineoplastic treatments are identified in this review as triggers of infectious mouth complications such as OC. This is related to the neutropenia resulting from such therapies, which in turn reduces the function of the oral environment and favors the emergence of this fungal pathological condition.
Most consulted authors report that the pathogenicity and virulence of OC depends on the state of immunosuppression of the patient subjected to antineoplastic therapies. In addition, immunosuppressed patients with cancer have an increased risk of disseminating OC to other regions of the body, like systemic circulation, which causes candidemia, with the consequent decrease in survival.
The prolongation or chronicity of OC is another characteristic of Candida infection. Leukemias or hematological tumors are associated with pseudomembranous and erythematous candidiasis, which are equally of chronic evolution and easy dissemination.
The revised literature suggests that the microscopic, macroscopic, and clinical characteristics of C. albicans and OC do not differ much from those observed in healthy patients; however, OC has been classified as a characteristic infection in cancer patients under antineoplastic treatment.