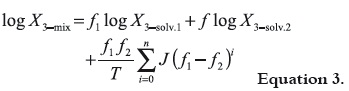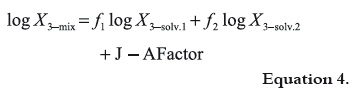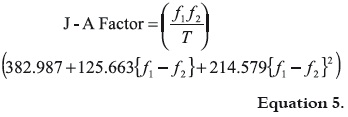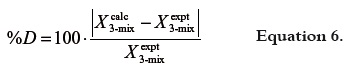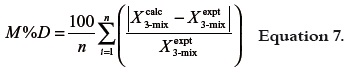Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.17 no.3 Medellín Sept./Dec. 2010
PHARMACEUTICAL INDUSTRY
PERFORMANCE OF THE JOUYBAN-ACREE MODEL FOR CORRELATING THE SOLUBILITY OF INDOMETHACIN AND ETHYLHEXYL TRIAZONE IN ETHYL ACETATE + ETHANOL MIXTURES
DESEMPEÑO DEL MODELO DE JOUYBAN-ACREE EN LA CORRELACIÓN DE LA SOLUBILIDAD DE INDOMETACINA Y ETILHEXIL TRIAZINA EN MEZCLAS ACETATO DE ETILO + ETANOL
Miller A. RUIDIAZ M.1; Sylvia J. RODRÍGUEZ D.1; Paula C. NEITA R.1; Diana M. CRISTANCHO B. 1; Fleming MARTÍNEZ R. 2
1 Grupo de Investigaciones Farmacéutico-Fisicoquímicas. Departamento de Farmacia. Facultad de Ciencias. Universidad Nacional de Colombia. A.A. 14490. Bogotá D.C., Colombia.
2 Grupo de Investigaciones Farmacéutico-Fisicoquímicas. Departamento de Farmacia. Facultad de Ciencias. Universidad Nacional de Colombia. A.A. 14490. Bogotá D.C., Colombia. fmartinezr@unal.edu.co.
ABSTRACT
This work reports the experimental volumetric properties and also the saturated solubility of indomethacin and ethylhexyl triazine in ethyl acetate + ethanol mixtures at 293.15 to 313.15 K and evaluates the validity of the Jouyban-Acree (J & A) model to correlate the solubility of these compounds in ethyl acetate + ethanol solvent mixtures. The solubility correlation is studied as a function of temperature and cosolvent composition. The J & A model requires only the experimental solubility values in the pure solvents at all the temperatures under study. The calculated values by using both compounds deviate as mean in 30% from experimental solubility values.
Keywords: Indomethacin, ethylhexyl triazone, solubility, ethanol, ethyl acetate, solvent mixtures, Jouyban-Acree equation.
RESUMEN
En este trabajo se evalúa la validez del modelo de Jouyban-Acree (J – A) para la correlación de la solubilidad de estos dos agentes de uso farmacéutico en mezclas acetato de etilo + etanol, en función de la composición solvente y de la temperatura, en el intervalo entre 293,15 y 313,15 K. El modelo J – A requiere únicamente de los valores experimentales de solubilidad de los fármacos en los solventes puros en función de la temperatura. Se encuentra que los valores obtenidos con los dos compuestos presentan desviaciones cercanas al 30% respecto a los valores experimentales de solubilidad.
Palabras clave: Indometacina, Etilhexil triazina, solubilidad, etanol, acetato de etilo, mezclas de solventes, ecuación de Jouyban-Acree.
INTRODUCTION
Indomethacin (IMC, molecular structure showed in figure 1) is an anti-inflammatory drug sometimes used in actual therapeutics (1), while ethylhexyl triazone (EHT, molecular structure showed in figure 2) is a sunscreen agent widely used in the formulation of skin care products (2, 3). Physicochemical properties of IMC and EHT have not been thoroughly studied. In this context, it is well known that several physicochemical properties such as, the solubility and occupied volumes by active ingredients and excipients in adequate solutions, are very important for pharmaceutical scientists, because they facilitate the processes associated to design and development of new products in the pharmaceutical and cosmetic industries (4).
On the other hand, ethyl acetate and ethanol have been widely used in drug microencapsulation processes (5). Moreover, ethyl acetate + ethanol binary system has been widely used as model mixed solvent for solubility studies of several drugs developed by Bustamante et al (6-13). Recently, Jouyban and Acree, 2007 (14) have developed a semi-empirical method intended to estimate drugs solubilities in this binary solvent system, whereas Ruidiaz and Martinez, 2009 (15) and Rodríguez et al., 2010 (16) have evaluated the usefulness of the Extended Hildebrand Solubility Approach to estimate the solubility of indomethacin and ethylhexyl triazone at 298.15 K in the same solvent system, respectively. Ultimately, Ruidiaz et al., 2010 have evaluated the volumetric behavior of this pharmaceutical model solvent system (17). For these reasons, the main objective of this study was to evaluate the usefulness of Jouyban-Acree model (14) to correlate the equilibrium solubility of two pharmaceutical compounds with great difference in molar mass and volume, namely, IMC and EHT, in binary mixtures conformed by ethyl acetate and ethanol as a function of the solvent composition and temperature.
THEORETICAL ASPECTS
Several methods to estimate the solubility in solvent mixtures have been reported in the pharmaceutical and chemical literature. Some of them have been challenged recently in the correlation of the equilibrium solubility of several drugs (18, 19).
As was already exposed (20), the simplest model to predict drug solubility in mixtures is the one based on the algebraic rule of mixing, which for semipolar compounds in binary mixtures takes the following form:
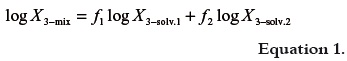
where X3-mix is the calculated solubility of solute in the mixture considered, X3-solv.1 is the solute solubility in the neat solvent 1, X3-solv.2 is the solute solubility in the neat solvent 2, and f1 and f2 are the volume fractions of both solvents in the mixture free of solute. The first one is calculated, by assuming volumes additivity as follows:
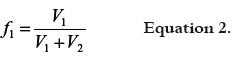
where, V1 and V2 are the volumes of solvents 1 and 2, respectively (21). It is clear that f2 is equal to 1 – f1. Nevertheless, it was found experimentally that the behavior of several lipophilic solutes deviate notoriously of this simple additive rule of solubility, in particular when the solvents used are amphiprotic. As good attempt to consider the deviations non taken into account by equation 1 Jouyban and Acree proposed the equation 3, where T is the absolute temperature and Ji are the respective polynomial coefficients. Ji coefficients present theoretical meaning because each one of them is a function of the interaction energies among two and three bodies, which in turn describe the attractions among the different molecules present in solution. Equation 3 is derivate from the equation originally proposed by Redlich and Kister, 1948 (22), and its development as well as its meaning has been described previously in the literature (23, 24).
Recently, Jouyban and Acree, 2007 (14) processed by regression analysis the solubility values (as mole fraction) of several drugs in AcOEt + EtOH mixtures reported in the literature (6-13), in front to equation 3, obtaining the equations 4 and 5,
where the J – A Factor is defined according to the following expression:
In equations 4 and 5 the solvent 1 is the one where the solubility is greatest between both neat solvents considered. As examples, for the solubility of caffeine in AcOEt + EtOH mixtures, AcOEt is the solvent 1 and EtOH is the solvent 2, whereas for the solubility of acetaminophen in the same solvent system, EtOH is the solvent 1 and AcOEt is the solvent 2 (14).
MATERIALS AND METHODS
Materials
In this investigation the following reagents and materials were used, indomethacin BP (25), ethylhexyl triazone obtained from BASF, ethyl acetate A.R. Merck (AcOEt), absolute ethanol A.R. Merck (EtOH), molecular sieve Merck (numbers 3 and 4, pore size 0.3 and 0.4 nm, respectively), and Durapore® 0.45 μm filters from Millipore Corp.
Solvent mixtures preparation
The dehydrated ethanol employed was maintained over molecular sieve (Merck Number 3, 0.3 nm in pore diameter) to obtain a dry solvent previously to prepare the solvent mixtures. The ethanol dryness was demonstrated by the respective density value obtained (0.7854 g cm–3 at 298.15 K), which was thus coincident with those reported in the literature (26, 27). All AcOEt + EtOH solvent mixtures were prepared in quantities of 10.00 g by mass using an Ohaus Pioneer TM PA214 analytical balance with sensitivity ± 0.1 mg, in mass fractions from 0.10 to 0.90 varying by 0.10, in order to study nine mixtures and both pure solvents.
Solubility determination
An excess of IMC or EHT was added to each organic solvent evaluated in stoppered dark glass f lasks. Solid-liquid mixtures were placed on thermostatic baths (Neslab RTE 10 Digital One Thermo Electron Company) kept at temperatures from 293.15 ± 0.05 K to 313.15 ± 0.05 K with sporadic stirring for at least three days to reach the solution equilibrium (this equilibrium time was established by quantifying the IMC or EHT concentration up to obtain constant values). Once at equilibrium, supernatant solutions were filtered (at isothermal conditions) to remove insoluble particles before the respective composition analyses. IMC or EHT concentrations were determined by mass balance by weighing a specified quantity of the respective saturated solution and allowing the solvent evaporation up to constant mass. All the solubility experiments were run at least in triplicate. In order to make the equivalence between volumetric and gravimetric concentration scales, the density of the saturated solutions was determined with a digital density meter (DMA 45 Anton Paar) connected to the same recirculating thermostatic baths.
Deviations calculation
As a deviation criterion between single experimental and calculated values by equations 1 and 5, the percentage deviations (%D) were calculated considering the unmodified solubility values according to following equation:
On similar way, as a general criterion of the usefulness of both equations the mean percentage deviations (M%D) were calculated by means of the equation 7, where n is the number of mixtures compositions considered.
RESULTS AND DISCUSSION
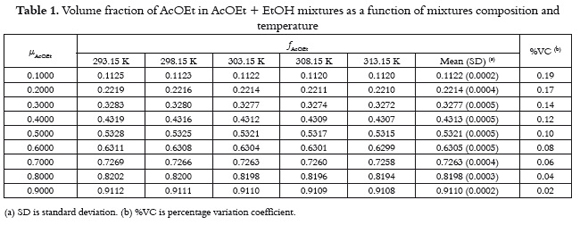
It is well known that the volumetric concentration scales depend on temperature varying according to their respective thermal-volume expansion coefficients (a). For this reason, table 1 shows the temperature dependence of volume fraction in AcOEt + EtOH mixtures with the mass composition varying in 0.10 in mass fraction (μAcOEt). The respective statistical description is also showed.
Although the a values for AcOEt and EtOH are slightly different, 1.397 x 10–3 K–1 and 1.123 x 10–3 K–1, respectively (17), the temperature dependence of f with temperature is relatively low, being in the nine cases lower than 0.19%, which for practical purposes is considered insignificant. Moreover, the mean values obtained are similar to those obtained at 303.15 K. For this reason, in challenging equations 1 to 5 the values obtained at this temperature were used on the same way as it was done in other similar investigations (28-30).
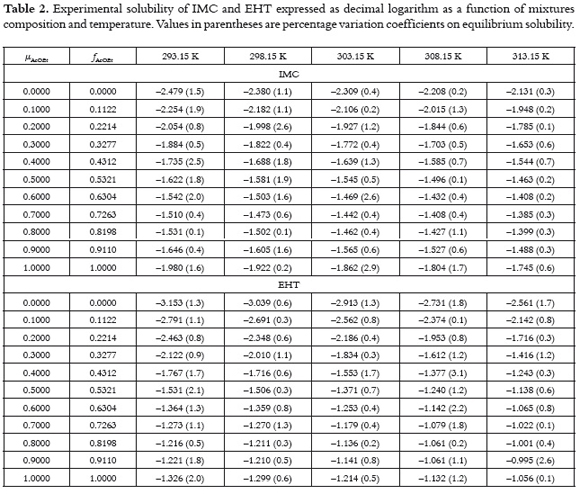
Table 2 shows the experimental values of equilibrium solubility for both pharmaceutical compounds expressed as decimal logarithms of mole fraction. The values used as input in equations 1 to 5 were those obtained in neat solvents at all temperatures.
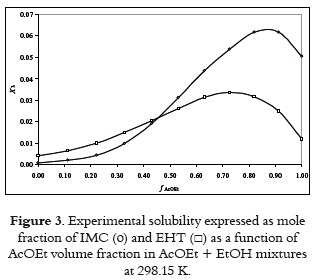
On the other hand, figure 3 shows the experimental solubility of both drugs at 298.15 K expressed as mole fraction. It is clear that maximum solubility is obtained in solvent mixtures instead of neat solvents, although the greatest solubility in neat solvents is obtained in AcOEt for both drugs. In this way, for these compounds the solvent 1 is AcOEt and the solvent 2 is EtOH.
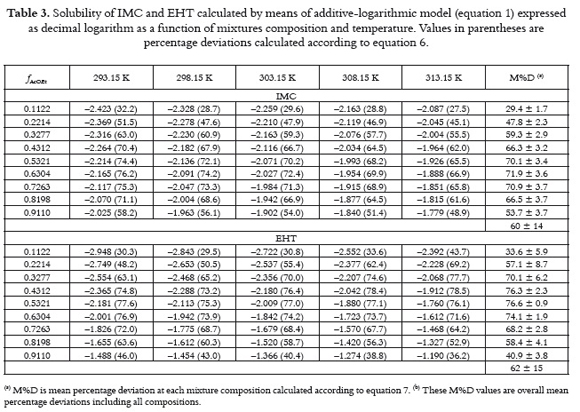
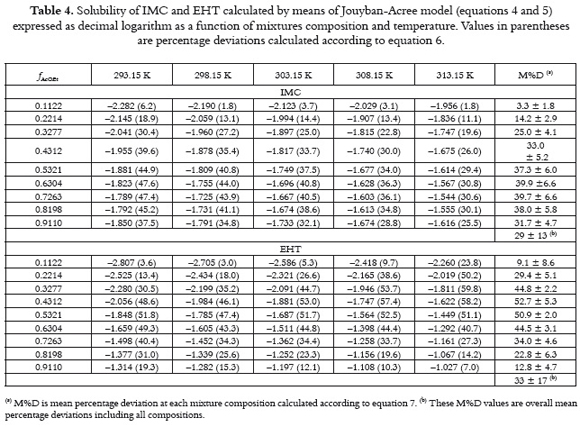
Tables 3 and 4 show the values of logarithmic solubility calculated by means of equations 1 and 4 as a function of mixtures composition and temperature for both drugs, respectively. Individual and group percentage deviations with respect to equilibrium solubilities are also showed in tables 3 and 4. It is important to note that these methods were selected for this study because they are the most simple among those described in the literature (18).
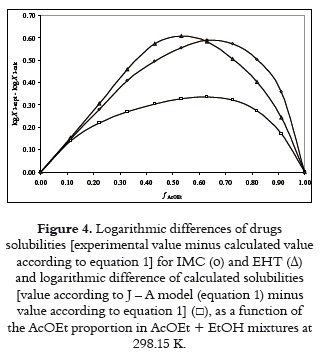
By comparing the predictive results obtained for both drugs it is clear that Jouban-Acree model (equations 4 and 5) is better than additive behavior (equation 1), because of their M%D values, namely, 29 ± 13% for IMC and 33 ± 17% for EHT in the first case, in front to 60 ± 14% for IMC and 62 ± 15% for EHT in the case of equation 1. Thus, J – A model would be useful if equilibrium solubility estimations within 30% in uncertainty are allowed. To see more clearly these effects, figure 4 shows the differences obtained between experimental solubilities for both drugs at 298.15 K in front to those calculated by means of equation 1. figure 4 also shows the differences obtained between equations 1 and 4 (and 5), respectively.
Figure 4 shows that differences obtained are positive in all cases and dependent on solvent composition. Thus, experimental solubilities for both compounds are greater than those predicted by equations 1 and 4 (and 5). It is interesting to note that the greatest experimental IMC solubility is found in the same mixture that J – A model predicts the maximum solubility, that is, near to 0.60 in volume fraction of AcOEt. Otherwise, the maximum solubility of EHT is found in the mixture with composition near to 0.50 in volume fraction of AcOEt.
Because the equation 4 (J – A model) is an extension of equation 1, figure 4 shows the excess factor of Jouyban-Acree (J – A Factor), which is equivalent to the logarithmic difference between calculated solubilities by means of both equations, and it is a global excess solubility function.
CONCLUSIONS
The generated solubility data of two drugs in ethyl acetate + ethanol mixtures at various temperatures extend the available database of solubility data of pharmaceuticals (31) which is in high demand in the industry. From all topics discussed previously it follows that IMC and EHT experimental solubilities present positive deviations in front to those predicted by the Jouyban-Acree model in the AcOEt + EtOH binary solvent system at all compositions studied. These estimation differences are within 30% as mean for both drugs which makes possible the use of the J – A model if these differences are allowed along the different stages of design and development of new products in the pharmaceutical and cosmetic industries.
ACKNOWLEDGEMENTS
The authors thank to the DIB the Universidad Nacional de Colombia (UNC) for the financial support and the Department of Pharmacy of UNC for facilitating the equipments and installations used in the experimental solubility determinations. Also to Prof. A. Jouyban of Tabriz University of Medical Sciences (Iran) for donating the bibliographic material required in this investigation.
REFERENCES
1. Raffa RB. Analgesic, antipyretic, and anti-inflammatory drugs. In: Gennaro AR, Editor. Remington: The Science and Practice of Pharmacy. 21 ed. Philadelphia, USA: Lippincott Williams & Wilkins; 2005. p. 1524–1542. [ Links ]
2. Tuchinda C, Lim HW, Osterwalder U, Rougier A. Novel emerging sunscreen technologies. Dermatol Clinics. 2006 Jan; 24 (1): 105–117. [ Links ]
3. Palm MD, O'Donoghue MN. Update on photoprotection. Dermatol Ther. 2007 Nov 28; 20 (5): 360–376. [ Links ]
4. Jiménez F, Martínez F. Una estrategia para la selección sistemática de vehículos en el diseño de formas farmacéuticas liquidas homogéneas. Rev Colomb Cienc Quím Farm. 1995 Dec; 24 (1): 19–23. [ Links ]
5. Tewes F, Boury F, Benoit JP. Biodegradable microspheres: Advances in production technology. In: Benita S, Editor. Microencapsulation: Methods and Industrial Applications. 2nd ed. New York, USA: Taylor & Francis roup, LLC; 2006. p. 1–53. [ Links ]
6. Bustamante P, Ochoa R, Reillo A, Escalera JB. Chameleonic effect of sulfanilamide and sulfamethazine in solvent mixtures. Solubility curves with two maxima. Chem Pharm Bull. 1994 Jun; 42 (5): 1129–1133. [ Links ]
7. Escalera JB, Bustamante P, Martin A. Predicting the solubility of drugs in solvent mixtures: multiple solubility maxima and the chameleonic effect. J Pharm Pharmacol. 1994 Mar; 46 (3): 172–176. [ Links ]
8. Bustamante P, Escalera B. Enthalpy and entropy contributions to the solubility of sulfamethoxypyridazine in solvent mixtures showing two solubility maxima. J Pharm Pharmacol. 1995 Jul; 47 (7): 550–555. [ Links ]
9. Romero S, Reillo A, Escalera B, Bustamante P. The behaviour of paracetamol in mixtures of aprotic and amphiprotic-aprotic solvents. Relationship of solubility curves to specific and nonspecific interactions. Chem Pharm Bull. 1996; 44 (5): 1061–1066. [ Links ]
10. Romero S, Escalera B, Bustamante P. Solubility behavior of polymorphs I and II of mefenamic acid in solvent mixtures. Int J Pharm. 1999 Feb 15; 178 (2): 193–202. [ Links ]
11. Jouyban A, Romero S, Chan HK, Clark BJ, Bustamante P. A cosolvency model to predict solubility of drugs at several temperatures from a limited number of solubility measurements. Chem Pharm Bull. 2002 May; 50 (5): 594–599. [ Links ]
12. Bustamante P, Navarro J, Romero S, Escalera B. Thermodynamic origin of the solubility profile of drugs showing one or two maxima against the polarity of aqueous and nonaqueous mixtures: Niflumic acid and caffeine. J Pharm Sci. 2002 Oct 4; 91 (3): 874–883. [ Links ]
13. Peña MA, Reillo A, Escalera B, Bustamante P. Solubility parameter of drugs for predicting the solubility profile type within a wide polarity range in solvent mixtures. Int J Pharm. 2006 Sep 14; 321 (1-2): 155–161. [ Links ]
14. Jouyban A, Acree Jr WE. Prediction of drug solubility in ethanolethyl acetate mixtures at various temperatures using the Jouyban-Acree model. J Drug Del Sci Tech. 2007; 17 (2): 159–160. [ Links ]
15. Ruidiaz MA, Martínez F. Método extendido de Hildebrand en la estimación de la solubilidad de la indometacina en mezclas acetato de etilo + etanol. Rev Colomb Quím. 2009; 38 (2): 235–247. [ Links ]
16. Rodríguez SJ, Cristancho DM, Neita PC, Vargas EF, Martínez F. Extended Hildebrand solubility approach in the solubility estimation of the sunscreen ethylhexyl triazone in ethyl acetate + ethanol mixtures. Lat Am J Pharm. 2010; 29 (7): 1113-1119. [ Links ]
17. Ruidiaz MA, Vargas EF, Martínez F. Study of some volumetric properties of the pharmaceutical model solvent system ethanol + ethyl acetate at several temperatures. Lat Am J Pharm. 2010; 29 (2): 306–312. [ Links ]
18. Jouyban-Gharamaleki A, Valaee L, Barzegar-Jalali M, Clark BJ, Acree Jr WE. Comparison of various cosolvency models for calculating solute solubility in water-cosolvent mixtures. Int J Pharm. 1999 Jan 15; 177 (1): 93–101. [ Links ]
19. Nokhodchi A, Shokri J, Barzegar-Jalali M, Ghafourian T. Prediction of benzodiazepines solubility using different cosolvency models. Farmaco 2002 Jul 4; 57 (7): 555–557. [ Links ]
20. Vargas EF, Manrique YJ, Pacheco DP, Torres NS, Martínez F. Desviaciones al modelo logarítmico-lineal en la solubilidad de ibuprofén y naproxén en mezclas cosolventes propilenoglicolagua. Quím Nova. 2007; 30 (8): 1945–1950. [ Links ]
21. Connors KA. Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy. Hoboken NJ: Wiley-Interscience; 2002. p. 61–66. [ Links ]
22. Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948 Feb; 40 (2): 345–348. [ Links ]
23. Acree Jr WE. Mathematical representation of thermodynamic properties. Part 2. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich-Kister mathematical representation from a two-body and three-body interactional mixing mode. Thermochim Acta 1992 Apr 2: 198 (1): 71–79. [ Links ]
24. Jouyban A, Chew NYK, Chan HK, Khoubnasabjafari M, Acree Jr WE. Solubility prediction of salicylic acid in water-ethanolpropylene glycol mixtures using Jouyban-Acree model. Pharmazie 2006 Apr; 61 (4): 318–331. [ Links ]
25. BP 1988. The British Pharmacopoeia, Vol. I. Her Majesty's Stationery Office. London, England; 1988. p. 310. [ Links ]
26. Resa JM, González C, Goenaga JM, Iglesias M. Temperature dependence of excess molar volumes of ethanol + water + ethyl acetate. J Solution Chem. 2004 Feb; 33 (2): 169–198. [ Links ]
27. Belda R, Herraez JV, Diez O. Rheological study and thermodynamic analysis of the binary system (water/ethanol): influence of concentration. Phys Chem Liq. 2004 Oct; 42 (5): 467–476. [ Links ]
28. Vargas E, Sosnik A, Martínez F. Aplicación del modelo de Jouyban-Acree para la estimación de la solubilidad del naproxeno en mezclas cosolventes etanol + agua. Lat Am J Pharm. 2008; 27 (5): 654–660. [ Links ]
29. Vargas EF, Barbosa H, Martínez F. Utilidad del modelo de Jouyban-Acree para la estimación de la solubilidad del ibuprofeno y el naproxeno en algunas mezclas cosolventes. Rev Ing Invest. 2008 Aug; 28 (2): 30–36. [ Links ]
30. Gantiva M, Yurquina A, Martínez F. Desempeño de los modelos de Yalkowsky & Roseman y de Jouyban & Acree en la estimación de la solubilidad del ketoprofeno en mezclas cosolventes etanol + agua. Vitae Rev Fac Quím Farm. 2009 Sept-Dec; 16 (3): 361–368. [ Links ]
31. Jouyban A. Handbook of Solubility Data for Pharmaceuticals. Boca Raton, USA: CRC Press, 2009. [ Links ]
Received: 26 March 2010
Accepted: 14 July 2010