Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Reumatología
versión impresa ISSN 0121-8123
Rev.Colomb.Reumatol. vol.23 no.2 Bogotá abr./jun 2016
Review Article
Usefulness of measuring common carotid intima-media thickness: Ultrasound diagnosis of sub-clinical atherosclerosis in rheumatic diseases. A literature review*
Medición del grosor de la íntima-media carotídea: utilidad y diagnóstico ecográfico de aterosclerosis subclínica en enfermedades reumáticas. Revisión de la literatura
Lina María Saldarriaga Rivera**, Lucio Ventura Ríos, Cristina Hernández Díaz, Carlos Pineda Villaseñor
Laboratorio de ultrasonido musculoesquelético y articular, Instituto Nacional de Rehabilitación, México D.F., México
* Please cite this article as: Saldarriaga Rivera LM, Ventura Ríos L, Hernández Díaz C, Pineda Villaseñor C. Medición del grosor de la íntima-media carotídea: utilidad y diagnóstico ecográfico de aterosclerosis subclínica en enfermedades reumáticas. Revisión de la literatura. Rev Colomb Reumatol. 2016;23:92-101.
** Corresponding author. E-mail address: vasculitisreumato@gmail.com (L.M. Saldarriaga Rivera).
Article history: Received 12 June 2015 Accepted 25 February 2016 Available online 9 September 2016
Abstract
Introduction: Patients with rheumatic diseases have an increased cardiovascular risk due to systemic inflammation and endothelial dysfunction, which promotes accelerated atherosclerosis. One way to measure cardiovascular risk, is through the carotid intima-media thickness which is an inexpensive and non-invasive predictor of cardiovascular events.
Objective: To examine the evidence to determine the usefulness of ultrasound in the diagnosis of sub-clinical atherosclerosis in rheumatic diseases assessed by carotid intima-media thickness (IMT).
Material and methods: A systematic literature search was performed, using electronic databases of PubMed, for articles published from January 2005 to May 2015, with no lan-guage restriction. Observational cohort studies that assessed the prevalence of sub-clinical atherosclerosis using the IMT were included.
Results: A total of 56 studies were identified for analysis, with almost all (95.7%) reporting an increased IMT in relation to the control group.
Conclusions: Patients with rheumatic diseases have an increased
cardiovascular risk assessed using IMT. This measurement, assessed bycarotid ultrasound, may help detect the risk of sub-clinical cardiovascular disease in these populations, allowing to establish a therapeutic strategy to reduce the risk of morbidity and mortality in these patients.
Keywords: Ultrasound, Intima-media, Carotid, Rheumatic diseases, Atherosclerosis, Cardiovascular risk.
Resumen
Introducción: Los pacientes con enfermedades reumáticas tienen un aumento del riesgo cardiovascular debido a inflamación sistémica y disfunción endotelial, lo que promueve una acelerada aterosclerosis. Una de las formas de medir el riesgo cardiovascular es a través del grosor de la íntima-media, evaluada por ultrasonido carotídeo, el cual es un predictor de acontecimientos cardiovasculares de bajo costo y naturaleza no invasiva.
Objetivo: Revisar la evidencia que describe la utilidad y valor del diagnóstico ecográfico de aterosclerosis subclínica en enfermedades reumáticas, evaluadas mediante el grosor de la íntima-media carotídea (GIMc).
Materiales y métodos: Se realizó una revisión de la literatura de la base de datos electrónica PubMed. Se incluyeron artículos desde enero de 2005 a mayo de 2015, sin restricción de idioma. Se incluyeron estudios observacionales de cohorte que evaluaron la prevalencia de aterosclerosis subclínica mediante la medición del GIMc y estudios de meta-análisis. Se verificó la calidad metodológica de los artículos y se extrajo la información relevante de cada uno.
Resultados: Se identificaron 56 artículos que cumplieron los requisitos. El 95,7% coincidió con el aumento del GIMc en relación con el grupo control, como marcador predictivo de aterosclerosis subclínica.
Conclusiones: Los pacientes con enfermedades reumáticas tienen un aumento del riesgo cardiovascular medido a través del GIMc, como lo muestran varios estudios. Esta medición realizada por ultrasonido carotídeo podría ayudar a detectar el riesgo de enfermedad cardiovascular subclínica en estas poblaciones, lo que permitiría al clínico implementar medidas terapéuticas para reducir el riesgo de morbimortalidad en estos pacientes.
Palabras clave: Ultrasonido, Íntima-media, Carótida, Enfermedades reumáticas, Aterosclerosis, Riesgo cardiovascular.
Introduction
Atherosclerosis is considered a pathological alteration of the arteries characterized by the abnormal deposit of lipids and fibrous tissue in the arterial walls, which disrupts the architecture and function of the vessels and reduces, variably, the blood flow to the myocardium.1,2 It is a progressive process that begins in adolescence, becoming clinical manifest at a later age or, on the contrary, it may occur in a subclinical manner.3
Systemic autoimmune diseases are related to a chronic inflammatory state, an increased cardiovascular risk and, therefore, to an increase in the rate of morbidity and mortality, both because of the active disease, and because of the prolonged use of steroids.4 Many of the cardiovascular risk factors, such as: smoking, arterial hypertension, dyslipidemia and altered vascular homeostasis, contribute to endothelial activation during the early stages of the development of atherosclerosis, resulting in an increase in the expression of adhesion molecules such as P-selectin, E-selectin, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on the endothelial cell surface, which leads to the union and, finally, promotes the adhesion of monocytes.5,6 After the transmigration in the vessel wall, the monocytes are converted into macrophages and subsequently into foam cells. The process continues with the accumulation of lipids, release of cytokines and proliferation of smooth muscle cells, generating the formation of atheromatous plaque.7,8
Endothelial activation, common in rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren's syndrome (SS), systemic sclerosis (SSc), gout, osteoarthritis (OA) and vasculitis, among others, is probably induced by autoantibodies. For the specific case of vasculitis, anti-neutrophil cytoplasmic antibodies (ANCA) and, in particular, those directed against proteinase 3 (PR3-ANCA), can activate the endothelial cells as demonstrated in vitro.9 It also occurs in vivo, due to the increased serum levels of vascular cell adhesion molecule-1 and E-selectin, which are involved in the process of systemic inflammation, contributing to the progression of cardiovascular damage.10 In the case of SLE, it is suggested that an alteration in lipid oxidation plays an important role in atherogenesis, contributing to the activation of the immune system, triggering inflammatory markers involved in the formation of the atheromatous plaque.11 In RA have been documented a variable number of factors for the development of atherogenesis, such as homocysteine, altered serum lipoprotein levels and prolonged pharmacological therapy. Recent findings indicate that the systemic inflammation may contribute to the development of atherosclerosis and confer an additional risk for cardiovascular death in patients with RA. The C-reactive protein has been considered as an independent prognostic marker for cardiovascular disease in these patients.12 For that purpose, the studies have demonstrated a close association between inflammation markers such as C-reactive protein, erythrocyte sedimentation rate and the markers for atheromatous cardiovascular disease (endothelial dysfunction and increased intima-media thickness of the carotid artery) in patients with these systemic autoimmune diseases, which significantly increases the prevalence of cardiovascular disease, as it has been reported in RA by 50%, similar to SLE with a risk of coronary heart disease, in women aged between 35 and 44 years, 50 times higher than in control subjects of the same age, and for ANCA-associated vasculitis with a higher than expected risk of 2.1 (CI 1.4-3.0), for cardiovascular events in the first 5 years of diagnosis.13,14
This review aims to examine the evidence that describes the usefulness and value of the echographic diagnosis of subclinical atherosclerosis in rheumatic diseases assessed by carotid IMT.
Measurement of the intima-media thickness
One of the ways to measure cardiovascular risk is through the intima-media thickness by ultrasonography, which is a predictor of cardiovascular events.15 Ultrasound B-mode is a non-invasive technique that allows the visualization of the walls of superficial arteries such as the carotid branches.15 IMT has been widely used in the evaluation of atherosclerotic progression; it has been demonstrated that the increased thickness is associated with a higher prevalence and severity of coronary and cerebrovascular diseases.16,17
Epidemiological studies and clinical-therapeutic works of regression with lipid-lowering drugs have established that the carotid IMT is a marker sensitive to changes, valid for assessing the progression and regression of atherosclerotic disease, which would be the most appropriate method to evaluate the efficacy of pharmacological and non-pharmacological interventions, since it provides objective data on the changes in the arterial wall, data of risk and health of a specific population, to favor the implementation of preventive measures to decrease morbidity and mortality and improve the prognosis and, consequently, the patients' quality oflife.18,19
Carotid ultrasound based on radiofrequency
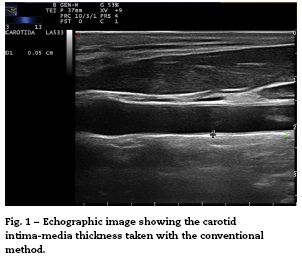
Conventionally, the measurement of the intima-media thickness is carried out by the manual tracing of the interfaces between the layers of arterial tissue (Fig. 1). It should be noted that this method requires high competence, appropriate training and a level of specialization in the technique. As a general rule, mainly cardiologists or radiologists perform this type of examination. Despite the conventional approach to evaluate the carotid IMT, there are still some issues that must be overcome, such as the wide intra and inter-observer variability, which depends on the experts judgment and on the time it takes to perform the measurements of the carotid IMT.20
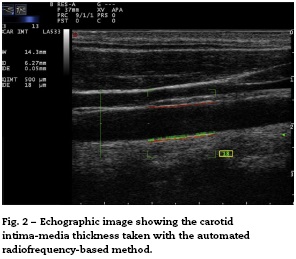
Recently, the technique of radiofrequency-based carotid ultrasound has been applied (Fig. 2). This method appears to be less dependent on the experience of the vascular ultrasound operator, since it provides an automatic measurement of the thickness of the interfaces of the arterial wall. Naredo et al. conducted a study whose objective was to assess the reliability of the automated radiofrequency-based ultrasound, comparing it with the B-mode of the conventional method, in patients with RA, demonstrating that the technique is valid, reproducible and reliable (intra-observer 0.61 and inter-observer 0.85) for the assessment of cardiovascular risk by the rheumatologist.21
Meanwhile, Di Geso et al. also compared both methods in 32 patients with chronic inflammatory rheumatic diseases. An expert cardiologist used the conventional manual method and a rheumatologist used the automated method for measuring the intima-media thickness. A good concordance between the two methods (0.69 and 0.77, for the right and left common carotid artery, respectively) was observed. Substantially good concordances were identified between the evaluators who adopted the conventional manual technique and the automated software.22
More recently, Naredo et al. conducted a prospective, longitudinal, multicenter study to compare the carotid IMT, evaluated by the automated method in 94 patients with RA treated with synthetic disease-modifying antirheumatic drugs (DMARDs) vs. biological DMARDs, compared with 94 controls. The carotid IMT was significantly greater in the patients with RA treated only with synthetic DMARDs than in the controls (591.4 vs. 562.1; p = 0.035) and in patients treated with synthetic plus biological DMARDs vs. controls (591.4 vs. 558.8; p = 0.040). The results suggest that carotid ultrasound can show significant differences between patients with RA, depending on the treatment they receive.23 The advantage of the automated method is that is faster than the conventional. The inter-observer variation for the detection of plaques is variable, and depends on the level of preparation of the sonographer and the quality of the ultrasound image.24
Materials and methods
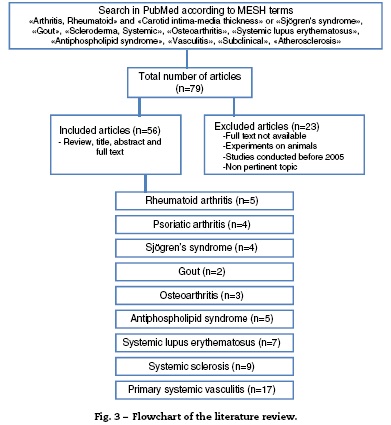
A literature review of observational cohort studies and meta-analyses published between January 2005 and May 2015, with no language restrictions, was conducted in the database PubMed, including finally those in Spanish, English and Portuguese. The methodological quality of the articles was verified and the relevant information of each one was extracted. The articles in which the studies were not conducted in humans, the topic was not pertinent, the full text was not available or that have been published before 2005 were excluded. For the search were used MESH terms that included "arthritis, rheumatoid" and "carotid intima-media thickness" or "Sjogren's syndrome", "gout", "scleroderma, systemic", "osteoarthritis", "systemic lupus erythematosus", "antiphospholipid syndrome", "vasculitis", "subclinical", "atherosclerosis".
The articles were selected by title and abstract, it was verified whether they were relevant and it was carried out the review of the full article. Using a collection format, from the selected articles were obtained relevant data regarding the carotid IMT and the formation of atheromatous plaques in each of the different rheumatic pathologies, compared with the control group (Fig. 3). The bibliographic citations of each article were reviewed looking for literature that was not recovered in the initial search.
Carotid intima-media thickness in rheumatic diseases
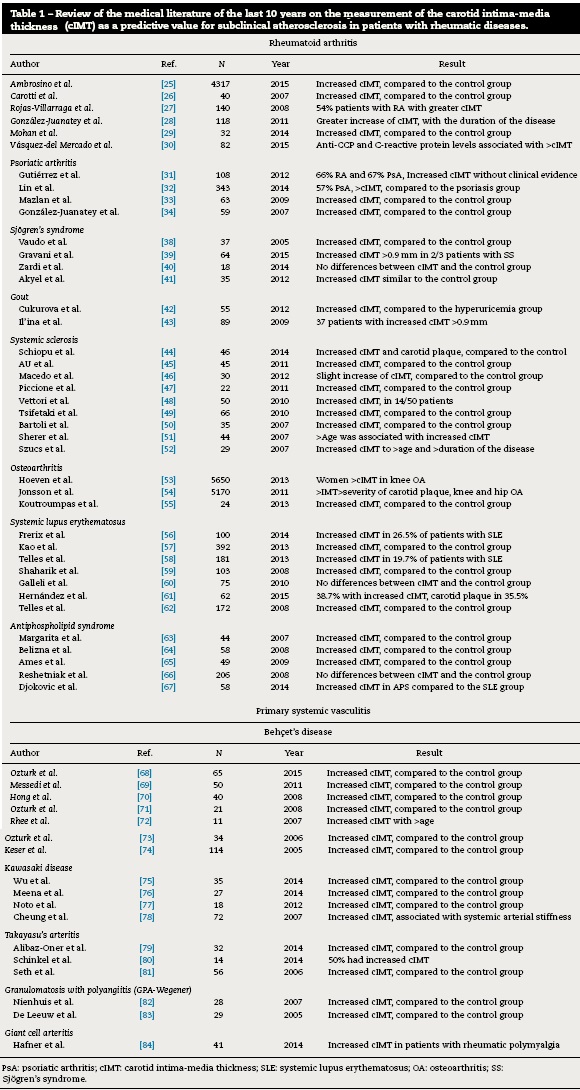
There are few studies in the literature regarding the measurement of carotid IMT in patients with rheumatic diseases, published in the last 10 years (Table 1 ).
Rheumatoid arthritis
Patients with RA have a reduced life expectancy and a high cardiovascular morbidity and mortality compared with the general population. A meta-analysis analyzed the RA in relation with the markers of cardiovascular risk and the presence of carotid plaques, finding 51 studies with data of the carotid IMT (3600 patients with RA and 3020 controls) and 35 studies that reported on the prevalence of atheromatous plaques (2859 patients with RA and 2303 controls). RA patients showed a significant increase of the carotid IMT compared to the controls. The men had a more serious inflammatory state.25 On the other hand, Carotti et al. found an accelerated atherosclerosis by increase of the carotid IMT in 40 patients with RA, greater than in the control group.26 A Colombian study showed that 54% of patients with RA had a carotid IMT greater than 0.91 mm correlating it with severe subclinical atherosclerosis, in the same way as the studies conducted by González-Juanatey, Mohan and Vázquez-Del Mercado, in which is also demonstrated an increase in the carotid IMT in this rheumatic pathology.27-30
Psoriatic arthritis
Gutiérrez et al. determined the prevalence of subclinical carotid atherosclerosis in 216 patients with RA or psoriatic arthritis, using the automated method; they observed that 66% with RA and 67% with psoriatic arthritis had a marked increase in the carotid IMT without clinical evidence.31 In another study was evaluated the relationship of the metabolic syndrome and the carotid IMT between 343 patients with psoriasis or psoriatic arthritis. 42.2% of patients with psoriasis and 57% with psoriatic arthritis had a higher prevalence of metabolic syndrome and measurements of the carotid IMT greater than patients with psoriasis, which contributes to an increased risk of cardiovascular disease.32
Mazlan et al. studied the carotid IMT and the cardiovascular risk factors in a population of 63 patients with psoriatic arthritis, using the conventional method, demonstrating a significant association between the cardiovascular risk and the increase in the carotid IMT, however, there was no association with the state of disease activity.33 González-Juanatey et al. demonstrated a higher prevalence of subclinical atherosclerosis in 59 patients with psoriatic arthritis, with respect to the controls.34
Sjögren's syndrome
There are some data on the risk of cardiovascular events in SS, in relation to the presence of valvular heart diseases, pericardial effusion, pulmonary hypertension, but few with measurement of the carotid IMT.35 Bartoloni et al. retrospectively analyzed a cohort of 1343 patients which was compared with a subgroup of 788 female patients, aged between 35 and 74 years and 4774 healthy women of the same age, finding a higher prevalence of cardiovascular risk factors such as systemic arterial hypertension and hypercholesterolemia.36 Likewise it is demonstrated in the study conducted by Kang et al., where hyperlipidemia and cardiac arrhythmias are the leading causes of comorbidity in patients with SS.37
In the last 10 years, 4 studies have been reported concerning the measurement of the carotid IMT. The first 2 were conducted by Vaudo et al. and Gravani et al. who demonstrated an increase in the carotid IMT compared to the control group.38,39 In contraposition with 2 studies carried out by Zardi et al. and Akyel et al. in which there was no significant difference in terms of the carotid IMT with respect to the control group.40,41
Gout
With regard to gouty arthritis, there are only 2 reports in the literature related with the evaluation of the carotid IMT and subclinical atherosclerosis. Cukurova et al. studied 55 patients with gout, finding an increase in the carotid IMT compared to the group of patients with asymptomatic hyperuricemia. Il'ina et al., in a population of 89 patients with gout, demonstrated that 37 had atheromatous plaques.42,43
Systemic sclerosis
Patients with SSc have a higher prevalence of formation of atheromatous plaques compared to the control group, as demonstrated by Schiopu et al. In contrast, in another study was demonstrated a slight increase in the carotid IMT with respect to the control group, but without statistical significance.44
In a systematic review were included 14 studies that evaluated the carotid IMT, reporting a high prevalence of subclinical atherosclerosis in these patients.45-47 In the last 10 years have been reported in the literature several studies that analyze the increase in the carotid IMT in patients with SSc, as a predictive marker of subclinical atherosclerosis. A study conducted by Vettori et al. reported subclinical atherosclerosis in 14/50 patients, correlating the carotid IMT with the old age and the prolonged use of corticosteroids.48 Tsifetaki et al. also described in a population of 60 SSc patients a higher prevalence of carotid IMT compared with the control group.49 Bartoli et al. demonstrated that the carotid IMT is an indicator of subclinical cardiovascular disease in their population of 35 patients with SSc, where the carotid IMT is greater when compared with the control group, like in the study conducted by Sherer et al.50,51
Szucs et al. demonstrated that the age and the duration of the disease are directly proportional to the increase of the carotid IMT.52
Osteoarthritis
There were found 3 studies, 2 of them with a representative sample. In the ROTTERDAM study that included 2372 men and 3278 women, it was observed an increase of the carotid IMT particularly in women with knee OA, and also increased formation of carotid plaques in patients with OA of the metacarpophalangeal and distal interphalangeal joints.53 The AGES-REYKJAVIK study analyzed a population of 2195 men and 2975 women with hip and knee OA, finding that patients with total joint replacements showed increased severity in terms of formation of carotid plaques.54 Finally, a small study conducted by Koutroumpas et al., showed in 24 patients an increase of the carotid IMT in erosive OA.55
Systemic lupus erythematosus
There are several studies that assess the increase of the carotid IMT in this disease, most of them have demonstrated its increase compared to the controls. The study conducted by Frerix et al., with a population of 100 patients with SLE and 90 with SSc, reveals the existence of subclinical atherosclerosis measured by the carotid IMT in 26.5% of patients with SLE, compared with 28.9% in the SSc group.56 Kao et al. demonstrate that patients who have had cardiovascular incidents have increased carotid IMT and formation of carotid plaques compared with the lupus population without previous cardiovascular events.57
Telles et al. evaluated the progression of the atherosclerosis in a cohort of 181 lupus patients, of whom 157 were reevaluated after 39 months, demonstrating in 19.7% an increase of the carotid IMT.58 The increase in the IMT was also detected in 15.4% of patients with lupus nephritis.59 Only one study did not show significant differences regarding the carotid IMT vs. the control group.60 As for the formation of atheromatous plaques, 2 studies reported its increase, being the most recent of them conducted by Hernández et al., which showed that 35.5% of the SLE population had carotid plaques.61,62
Antiphospholipid syndrome
With respect to this syndrome, only 5 publications were found, 3 of them reported a marked increase in the carotid IMT compared to the control group, in contraposition with the study conducted by Reshetniak et al., which did not show significant differences.63-66. In a comparative study between antiphospholipid syndrome and SLE, was evidenced that the antiphospholipid syndrome exhibits a greater increase of the carotid IMT (48.3%) than SLE, however, no significant relation-ship was established between the type of antiphospholipid antibodies and the carotid IMT changes.67
Primary systemic vasculitis
In relation to primary systemic vasculitis, there are few studies. The most studied pathologies in the last 10 years, in order of frequency are: Behcet's disease,68-74 Kawasaki disease,75-78 Takayasu's arteritis,79-81 GPA-Wegener and, finally, giant cell arteritis.82,83 For Takayasu's arteritis, a study conducted by Seth et al., suggests that the carotid IMT might be a possible marker of disease activity. In this study, 74 common carotid arteries were evaluated in 37 patients with Takayasu's arteritis and 28 healthy controls, correlating their data with the presence of activity according to clinical criteria. The results found were a greater carotid IMT in 59% of the studied population, being prevalent in patients who exhibited dis-ease activity, having the measurement of the carotid IMT a sensitivity of 82% and a specificity of 60%, as an activity marker.77
Studies with ANCA-associated small vesselvasculitisof the eosinophilic granulomatosis with polyangiitis type (Churg-Strauss) and microscopic polyangiitis are not found. In all studies analyzed there is a marked increase in the carotid IMT compared to the control group.84
Conclusion
Patients with rheumatic diseases have an increased cardiovascular risk assessed by the carotid IMT, as demonstrated by several studies. This measurement could help to detect the risk of subclinical cardiovascular disease in these populations, which would allow the rheumatologist, in the clinical practice, to implement opportune therapeutic measures to reduce the morbidity and mortality, which already are increased. The automated radiofrequency-based ultrasound technique is still little used in rheumatology, however, it has been demonstrated that it is useful, valid, accessible, reproducible and reliable, so it is expected that in the future the evaluation of cardiovascular risk in rheumatic patients will be part of the quotidian assessment.
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
References
1. Suárez Loaiza J. Fisiopatología de la aterosclerosis. Rev Costarric Cardiol. 2001;3:54-63. [ Links ]
2. Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115-26. [ Links ]
3. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135-43. [ Links ]
4. Amaya-Amaya J, Montoya-Sánchez L, Rojas-Villarraga A. Cardiovascular involvement in autoimmune diseases. Biomed Res Int. 2014;3673:367359, http://dx.doi.org/10.1155/ 2014/367359. [ Links ]
5. Filer AD, Gardner-Medwin JM, Thambyrajah J, Raza K, Carruthers DM, Stevens RJ, et al Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis. 2003;62:162-7. [ Links ]
6. Booth AD, Wallace S, McEniery CM, Yasmin, Brown J, Jayne DR, et al. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum. 2004;50:581-8. [ Links ]
7. Morgan MD, Turnbull J, Selamet U, Kaur-Hayer M, Nightingale P, Ferro CJ, et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum. 2009;60:3493-500. [ Links ]
8. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251-62. [ Links ]
9. Faia KL, Davis WP, Marone AJ, Foxall TL. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in hamster aortic atherosclerosis: correlation with in-situ zymography. Atherosclerosis. 2002;160:325-37. [ Links ]
10. Monach PA. Biomarkers in vasculitis. Curr Opi Rheumatol. 2014;26:24-30. [ Links ]
11. Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, Du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331-7. [ Links ]
12. Corrales A, Dessein PH, Tsang L, Pina T, Blanco R, Gonzalez-Juanatey C, et al. Carotid artery plaque in women with rheumatoid arthritis and low estimated cardiovascular disease risk: a cross-sectional study. Arthritis Res Ther. 2015;17:55. [ Links ]
13. Faurschou M, Mellemkjaer L, Sorensen IJ, Thomsen BS, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum. 2009;60:1187-92. [ Links ]
14. Karrar A, Sequeira W, Block JA. Coronary artery disease in systemic lupus erythematosus: a review of the literature. Semin Arthritis Rheum. 2001;30:436-43. [ Links ]
15. Chain S, Luciardi HL, Feldman G, Valberdi A. El espesor íntima-media carotídeo, un marcador de ateroesclerosis subclínica y riesgo cardiovascular. Importancia de su valoración y dificultades en su interpretación. Rev Fed Arg Cardiol. 2005;34:392-402. [ Links ]
16. Carrizo A, Tazar J, Mendia A, Sánchez N, Bossi A. Correlación del espesor íntima-media de arterias carótidas con parámetros ecocardiográficos, factores de riesgos y eventos cardiovasculares. Insuf Card. 2013;8:112-8. [ Links ]
17. González A, Arrieta T, Solís L, Pérez M, Ramírez M. Estudio con ultrasonido Doppler color en la caracterización de la enfermedad ateroesclerótica carotídea oclusiva asintomática. Rev Chil Radiol. 2009;15:110-21. [ Links ]
18. Graua M, Subiranaa I, Agisb D, Ramosd R, Basagaüab X, Martid R, et al. Grosor íntima-media carotídeo en población española: valores de referencia y asociación con los factores de riesgo cardiovascular. Rev Esp Cardiol. 2012;65:1086-93. [ Links ]
19. Baldassarre D, Nyyssónen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ, et al., IMPROVE study group. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thicknes in a European population: the IMPROV study. Eur Heart J. 2010;31:614-22. [ Links ]
20. Hoeks AP, Willekes C, Boutouyrie P, Brands PJ, Willinger JM, Reneman RS. Automated detection of local artery well thickness based on M-line signal processing. Ultrasound Med Biol. 1997;23:1017-23. [ Links ]
21. Naredo E, Moller I, Gutiérrez M, Bong D, Cobo T, Corominas H, et al. Multi-examiner reliability of automated radio frequency-based ultrasound measurements of common carotid intima-media thickness in rheumatoid arthritis. Rheumatology (Oxford). 2011;50:1860-4. [ Links ]
22. Di Geso L, Zardi EM, Afeltra A, Salaffi F, Carotti M, Gutiérrez M, et al. Comparison between conventional and automated software-guide ultrasound assessment of bilateral common carotids intima-media thickness in patients with rheumatic diseases. Clin Rheumatol. 2012;31:881-4. [ Links ]
23. Naredo E, Moller I, Corrales A, Bong DA, Cobo-Ibanez T, Corominas H, et al. Automated radiofrequency-based US measurement of common carotid intimamedia thickness in RA patients treated with synthetic vs synthetic and biologic DMARDs. Rheumatology. 2013;52:376-81. [ Links ]
24. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An 80 update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim. Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75-80. [ Links ]
25. Ambrosino P, Lupoli R, di Minno A, Tasso M, Peluso R, di Minno MN. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost. 2015;113:916-30. [ Links ]
26. Carotti M, Salaffi F, Mangiacotti M, Cerioni A, Giuseppetti GM, Grassi W. Atherosclerosis in rheumatoid arthritis: the role of high-resolution B mode ultrasound in the measurement of the arterial intima-media thickness. Reumatismo. 2007;59:38-49. [ Links ]
27. Rojas-Villarraga A, Ortega-Hernández OD, Gómez LF, Pardo AL, López-Guzmán S, Arango-Ferreira C, et al. Risk factors associated with different stages of atherosclerosis in Colombian patients with rheumatoid arthritis. Semin Arthritis Rheum. 2008;38:71-82. [ Links ]
28. González-Juanatey, Llorca J, González-Gay MA. Correlation between endothelial function and carotid atherosclerosis in rheumatoid arthritis patients with long-standing disease. Arthritis Res Ther. 2011;13:101. [ Links ]
29. Mohan A, Sada S, Siddhartha BK, Sarma KVS, Devi V, Srinivasa PVLN, et al. Subclinical atherosclerosis in patients with rheumatoid arthritis by utilizing carotid intima-media thickness as a surrogate marker. Indian J Med Res. 2014;140:379-86. [ Links ]
30. Vázquez-Del Mercado M, Nunez-Atahualpa L, Figueroa-Sánchez M, Gómez-Barnielos E, Rocha-Muiioz AD, Martín-Márquez BT, et al. Serum levels of anticyclic citrullinated peptide antibodies, interleukin-6, tumor necrosis factor-a, and C-reactive protein are associated with increased carotid intima-media thickness: a cross-sectional analysis of a cohort of rheumatoid arthritis patients without cardiovascular risk factors. Biomed Res Int. 2015;2015:342-649. [ Links ]
31. Gutiérrez M, Naredo E, di Geso L, Moller I, Lagnocco A, Salaffi F, et al. Prevalence of sub-clinical carotid atherosclerosis in patients with rheumatoid arthritis and psoriatic arthritis: a new automated radiofrequency-based ultrasound measurement of common intima-media thickness. Ann Rheum Dis. 2014;71(S3):301-2. [ Links ]
32. Lin YC, Dalal D, Churton S, Brennan DM, Korman NJ, Kim ES, et al. Relationship between metabolic syndrome and carotid intima-media thickness: cross-sectional comparison between psoriasis and psoriatic arthritis. Arthritis Care Res (Hoboken). 2014;66:97-103. [ Links ]
33. Mazlan SA, bin Mohamed Said MS, Hussein H, binti Shamsuddin K, Shah SA, Basri H. A study of intima media thickness and their cardiovascular risk factors in patients with psoriatic arthritis. Acta Med (Hradec Kralove). 2009;52:107-16. [ Links ]
34. González-Juanatey C, Llorca J, Amigo-Díaz E, Dierssen T, Martin J, González-Gay MA. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074-80. [ Links ]
35. Vassiliou VA, Moyssakis I, Boki KA, Moutsopoulos HM. Is the heart affected in primary Sjögren's syndrome? An echocardiographic study. Clin Exp Rheumatol. 2008;26:109-12. [ Links ]
36. Bartoloni E, Baldini C, Schillaci G, Quartuccio L, Priori R, Carubbi F, et al. Cardiovascular disease risk burden in primary Sjögren's syndrome: results of a population-based multicentre cohort study. J Intern Med. 2015;278:185-92. [ Links ]
37. Kang JH, Lin HC. Comorbidities in patients with primary Sjogren's syndrome: a registry-based case-control study. J Rheumatol. 2010;37:1188-94. [ Links ]
38. Vaudo G, Bocci EB, Shoenfeld Y, Schillaci G, Wu R, del Papa N, et al. Precocious intima-media thickening in patients with primary Sjögren's syndrome. Arthritis Rheum. 2005;52: 3890-7. [ Links ]
39. Gravani F, Papadaki I, Antypa E, Nezos A, Masselou K, Ioakeimidis D, et al. Subclinical atherosclerosis and impaired bone health in patients with primary Sjogren's syndrome: prevalence, clinical and laboratory associations. Arthritis Res Ther. 2015:17-99. [ Links ]
40. Zardi EM, Sambataro G, Basta F, Margiotta DP, Afeltra AM. Subclinical carotid atherosclerosis in elderly patients with primary Sjögren syndrome: a duplex Doppler sonographic study. Int J Immunopathol Pharmacol. 2014;27:645-51. [ Links ]
41. Akyel A, Tavil Y, Yayla C, Tufan A, Kaya A, Tezcan ME, et al. Endothelial dysfunction in primary Sjögren syndrome. West Indian Med J. 2012;61:870-2. [ Links ]
42. gukurova S, Pamuk ÓN, Ünlü E, Pamuk GE, gakir N. Subclinical atherosclerosis in gouty arthritis patients: a comparative study. Rheumatol Int. 2012;32:1769-73. [ Links ]
43. Il'ina AE, Varfolomeeva EI, Volkov AV, Mach ES, Aleksandrova EN, Novikov AA, et al. Relationship between the intima-media complex thickness, the risk factors of cardiovascular diseases, and the level of C-reactive protein in gouty patients. Ter Arkh. 2009;81:45-9. [ Links ]
44. Schiopu E, Au KM, McMahon MA, Kaplan MJ, Divekar A, Singh RR, et al. Prevalence of subclinical atherosclerosis is increased in systemic sclerosis and is associated with serum proteins: a cross-sectional, controlled study of carotid ultrasound. Rheumatology (Oxford). 2014;53:704-13. [ Links ]
45. Au K, Singh MK, Bodukam V, Bae S, Maranian P, Ogawa R, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum. 2011;63:2078-90. [ Links ]
46. Macedo R, Andretta M, Albers C, Skare T, Ribas-Filho JM, Czeczko NG. Evaluation of carotid artery intima-media complex thickness as a marker of vascular damage secondary to accelerated atherogenesis in progressive systemic sclerosis. Rev Col Bras Cir. 2012;39:10-5. [ Links ]
47. Piccione MC, Bagnato G, Zito C, di Bella G, Caliri A, Catalano M, et al. Early identification of vascular damage in patients with systemic sclerosis. Angiology. 2011;62:338-43. [ Links ]
48. Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol. 2010;39:485-9. [ Links ]
49. Tsifetaki N, Georgiadis AN, Alamanos Y, Fanis S, Argyropoulou MI, Drosos AA. Subclinical atherosclerosis in scleroderma patients Scand J Rheumatol. 2010;39:326-9. [ Links ]
50. Bartoli F, Blagojevic J, Bacci M, Fiori G, Tempestini A, Conforti ML, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci. 2007;1108:283-90. [ Links ]
51. Sherer Y, Cerinic MM, Bartoli F, Blagojevic J, Conforti ML, Gilburd B, et al. Early atherosclerosis and autoantibodies to heat-shock proteins and oxidized LD in systemic sclerosis. Ann N Y Acad Sci. 2007;1108:259-67. [ Links ]
52. Szucs G, Tímár O, Szekanecz Z, Dér H, Kerekes G, Szamosi S, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis relevance for prevention of vascular complications. Rheumatology (Oxford). 2007;46:759-62. [ Links ]
53. Hoeven TA, Kavousi M, Clockaerts S, Kerkhof HJ, van Meurs JB, Franco O, et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis. 2013;72:646-51. [ Links ]
54. Jonsson H, Helgadottir GP, Aspelund T, Eiriksdottir G, Sigurdsson S, Siggeirsdottir K, et al. The presence of total knee or hip replacements due to osteoarthritis enhances the positive association between hand osteoarthritis and atherosclerosis in women: the AGES-Reykjavik study. Ann Rheum Dis. 2011;70:1087-90. [ Links ]
55. Koutroumpas A, Giannoukas A, Zintzaras E, Exarchou E, Baliakos A, Makaritsis K, et al. Erosive hand osteoarthritis is associated with subclinical atherosclerosis and endothelial dysfunction. Int J Biomed Sci. 2013;9:217-23. [ Links ]
56. Frerix M, Stegbauer J, Kreuter A, Weiner SM. Atherosclerotic plaques occur in absence of intima-media thickening in both systemic sclerosis and systemic lupus erythematosus: a duplexsonography study of carotid and femoral arteries and follow-up for cardiovascular events. Arthritis Res Ther. 2014;16:R54. [ Links ]
57. Kao AH, Lertratanakul A, Elliott JR, Sattar A, Santelices L, Shaw P, et al. Relation of carotid intima-media thickness and plaque with incident cardiovascular events in women with systemic lupus erythematosus. Am J Cardiol. 2013;112:1025-32. [ Links ]
58. Telles RW, Lanna CC, Sousa AJ, Navarro TP, Souza FL, Rodrigues LA, et al. Progression of carotid atherosclerosis in patients with systemic lupus erythematosus. Clin Rheumatol. 2013;32:1293-300. [ Links ]
59. Shaharir SS, Mohamed Said MS, Kong NC. Predictors of thickened carotid intima media thickness among well controlled lupus nephritis patients in a Malaysian tertiary centre. Reumatismo. 2012;64:341-9. [ Links ]
60. Gallelli B, Burdick L, Quaglini S, Banfi G, Novembrino C, Bamonti F, et al. Carotid plaques in patients with long-term lupus nephritis. Clin Exp Rheumatol. 2010;28:386-92. [ Links ]
61. Hernández Y, Guibert ZM, Reyes GA. Correlación de las cifras de proteína C reactiva y aterosclerosis en pacientes con lupus eritematoso sistémico. Rev Cuba Reumatol. 2015;17:126-31. [ Links ]
62. Telles RW, Lanna CC, Ferreira GA, Souza AJ, Navarro TP, Ribeiro AL. Carotid atherosclerotic alterations in systemic lupus erythematosus patients treated at a Brazilian university setting. Lupus. 2008;17:105-13. [ Links ]
63. Margarita A, Batuca J, Scenna G, Alves JD, López L, Iannaccone L, et al. Subclinical atherosclerosis in primary antiphospholipid syndrome. Ann N Y Acad Sci. 2007;1108:475-80. [ Links ]
64. Belizna CC, Richard V, Primard E, Kerleau JM, Cailleux N, Louvel JP, et al. Early atherom in primary and secondary antiphospholipid syndrome: an intrinsic finding. Semin Arthritis Rheum. 2008;37:373-80. [ Links ]
65. Ames PR, Antinolfi I, Scenna G, Gaeta G, Margaglione M, Margarita A. Atherosclerosis in thrombotic primary antiphospholipid syndrome. J Thromb Haemost. 2009;7:537-42. [ Links ]
66. Reshetniak TM, Seredavkina NV, Mach ES, Aleksandrova EN, Novikov AA, Cherkasova MV, et al. Subclinical and clinical manifestations of atherosclerosis in antiphospholipid syndrome. Ter Arkh. 2008;80:60-7. [ Links ]
67. Djokovic A, Stojanovich LJ, Stanisavljevic N, Bisenic V, Radovanovic S, Soldatovic I, et al. Does the presence of secondary antiphospholipi syndrome in patients with systemic lupus erythematodes accelerate carotid arteries intima-media thickness changes? Rheumatol Int. 2014;34:321-7. [ Links ]
68. Ozturk C, Balta S, Balta I, Demirkol S, Celik T, Turker T, et al. Neutrophil-lymphocyte ratio and carotid-intima media thickness in patients with Behcet disease without cardiovascular involvement. Angiology. 2015;66:291-6. [ Links ]
69. Messedi M, Frigui M, Ben Mahfoudh K, Feki H, Ben Mahfoudh ST, Mnif J, et al. Intima-media thickness of carotid artery in patients with Behcet's disease. Arch Med Res. 2011;42:398-404. [ Links ]
70. Hong SN, Park JC, Yoon NS, Lee SR, Kim KH, Hong YJ, et al. Carotid artery intima-media thickness in Behcet's disease patients without significant cardiovascular involvement. Korean J Intern Med. 2008;23:87-3. [ Links ]
71. Oztürk MA, Unverdi S, Oktar SO, Bukan N, Gülbahar O, Ureten K, et al. Vascular endothelial growth factor and carotid intima-media thickness in patients with Behcet's disease. Clin Rheumatol. 2008;27:961-6. [ Links ]
72. Rhee MY, Chang HK, Kim SK. Intima-media thickness and arterial stiffness of carotid artery in Korean patients with Behcet's disease. J Korean Med Sci. 2007;22:387-92. [ Links ]
73. Oztürk MA Oktar SO, Unverdi S, Ureten K, Góker B, Haznedaroglu S, et al. Morphologic evidence of subclinical atherosclerosis obtained by carotid ultrasonography in patients with Behcet's disease. Rheumatol Int. 2006;26:867-72. [ Links ]
74. Keser G, Aksu K, Tamsel S, Ozmen M, Kitapcioglu G, Kabaroglu C, et al. Increased thickness of the carotid artery intima-media assessed by ultrasonography in Behcet's disease. Clin Exp Rheumatol. 2005;23(S38):71-6. [ Links ]
75. Wu TH, Kuo HC, Tain YL, Lin KM, Kuo HC, Chien SJ. Common carotid artery intima-media thickness is useful for diagnosis of the acute stage of Kawasaki disease. BMC Pediatr. 2014;14:98. [ Links ]
76. Meena RS, Rohit M, Gupta A, Singh S. Carotid intima-media thickness in children with Kawasaki disease. Rheumatol Int. 2014;34:1117-21. [ Links ]
77. Noto N, Okada T, Abe Y, Miyashita M, Kanamaru H, Karasawa K, et al. Characteristics of earlier atherosclerotic involvement in adolescent patients with Kawasaki disease and coronary artery lesions: significance of gray scale median on B-mode ultrasound. Atherosclerosis. 2012;222:106-9. [ Links ]
78. Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92:43-7. [ Links ]
79. Alibaz-Oner F, Yurdakul S, Aytekin S, Direskeneli H. Impaired endothelial function in patients with Takayasu's arteritis. Acta Cardiol. 2014;69:45-9. [ Links ]
80. Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;15:541-6. [ Links ]
81. Seth S, Goyal NK, Jagia P, Gulati G, Karthikeyan G, Sharma S, et al. Carotid intima-medial thickness as a marker of disease activity in Takayasu's arteritis. Int J Cardiol. 2006;108:385-90. [ Links ]
82. Nienhuis HL, de Leeuw K, Smit AJ, Bijzet J, Stegeman CA, Kallenberg CG, et al. Enhanced endothelium-dependent microvascular responses in patients with Wegener's granulomatosis. J Rheumatol. 2007;34:1875-81. [ Links ]
83. De Leeuw K, Sanders JS, Stegeman CA, Smit A, Kallenberg CG, Bijl M. Accelerated atherosclerosis in patients with Wegener's granulomatosis. Ann Rheum Dis. 2005;64:75-59. [ Links ]
84. Hafner F, Haas E, Belaj K, Froehlich H, Gary T, Eller P, et al. Endothelial function and carotid intima-media thickness in giant-cell arteritis. Eur J Clin Invest. 2014;44:249-56. [ Links ]











 texto en
texto en