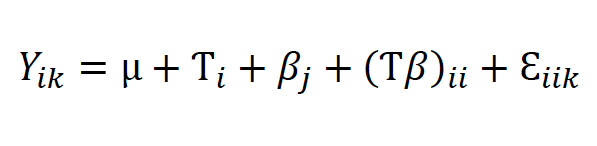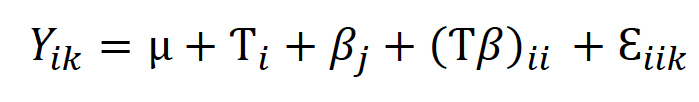Introduction
Arrow cane, Gynerium sagittatum (Aubl.) P. Beauv. (Poaceae) is the source of many handicrafts for the Zenú indigenous communities settled in the flatlands of Córdoba and Sucre, Colombia’s North Coast (Pérez, 1978; Uribe, 1982). Zenú craftsmanship is worldwide known because of its beauty and tradition; one of the best known is the sombrero vueltiao (a traditional hat), a Colombian cultural symbol commercially protected by the designation of origin (Superintendencia de Industria y Comercio [SIC], 2017). Aboriginal Zenú communities are legally organized in an indigenous reservation called RISAS (Resguardo Indígena Zenú de San Andrés de Sotavento) with around 18,000 people and 5,000 families economically depending on arrow cane artisanship work (Departamento Administrativo Nacional de Estadística, 2005; Serpa, 2000; Valencia, 1987).
The demand for arrow cane products has increased in the last years because of more frequent tourists and national and international handicraft sales (Artesanías de Colombia, 2019). Since there are no commercial arrow cane crops, fiber for manufacturing is extracted from natural plant populations, which have decreased around 50% in the past 20 years (Araméndiz et al., 2005). Reduction in arrow cane natural populations not only negatively affects artisans by increasing fiber costs and reducing profit from product sales but also has a significant impact on wetland ecosystems associated with arrow cane (López & Suárez, 2018). Radial plant growth from a single culm forms a niche for many insects, amphibians, and other animal species to complete their life cycle. Additionally, the plant root system is a natural barrier against soil erosion that preserves the ecosystem (Kalliola et al., 1992). Alternatives for providing arrow cane natural fiber to the handicraft industry are necessary not only to preserve the millenarian Zenú artisan legacy and Colombian cultural tradition but also to reduce the negative impact of the arrow cane manufacturing activity on the environment.
Several studies on clonal propagation of arrow cane have intended to produce plant material for commercial crop activities. A positional cutting evaluation found that horizontally planted cuttings resulted in more rooted plants (Suárez Padrón, 2020). End base immersion in auxin (NAA and IBA) treatments (40-120 mg/L) increased rooting percentage in 40 cm-long vertically planted cuttings (Hernández Murillo et al., 2005). Despite the results in rooting percentages, the length of the cutting needed for planting (> 30 cm) makes large-scale propagation inefficient using this method (Hernández Murillo et al., 2005).
Micropropagation is a clonal propagation technique that aseptically propagates plants in vitro using culture media as substrate under controlled environmental conditions (Cobo et al., 2018; Nunes et al., 2018; Singh et al., 2019; Tisarum et al., 2018). To approach the challenge of producing plant material for commercial planting of arrow cane (G. sagittatum), the Plant Biotechnology Research Group (GIBV, for its acronym in Spanish) at the Universidad de Córdoba has worked to develop a micropropagation protocol for arrow cane cv. Criolla (López & Suárez, 2018; Pastrana & Suárez, 2009; Suárez et al., 2009, 2017). Efforts are now focused on expanding micropropagation to different cultivars other than Criolla and lowering plant micropropagation costs to make arrow cane production more environmentally friendly.
Micropropagation using a double-phase medium system combines the use of semisolid and liquid media in a single recipient to allow the continuous growth of the explant, with no subcultures. Instead, nutrient supply is refreshed by periodically adding a new liquid phase, and tissue stress is reduced by maintaining cultures in the same recipient until transfer to the next stage of the protocol (Scherwinski- Pereira et al., 2012). This technique is expected to reduce costs by lowering the use of gelling agents and allow better plant growth by reducing the number of subcultures and therefore plant stress. The present work aims to evaluate the micropropagation response of three arrow cane cultivars using a double phase medium during the multiplication stage.
Material and methods
In vitro establishment
We obtained mother plants from field-grown shoots of Martinera and Criolla 1 cultivars planted at the Universidad de Córdoba Experimental Field. Isolated shoots were washed with tap water to remove soil and transferred into plastic pots (48.5 × 60.0 cm) filled with a peat-sand (1:1) substrate mix. We maintained plants in a shade house with 50% light and twice-a-day sprinkle irrigation (2 min each). Weekly alternate treatment with Mancozeb (4 g/L) and Benzoimidazol (6 g/L) was sprayed to prevent fungal attacks. Axillary or basal shoots emerging from planted shoots were isolated and used as an explant source for in vitro establishment. Leaves and shaft material were removed from stem sections (1 cm diameter) and sectioned at 3 cm long with a single axillary bud in each section. Portions were washed for 1 h with running water and then surface-disinfected in a sodium hypochlorite solution (1.25% active chlorine) with two drops of Tween 20® for 20 min while stirring continuously.
After that, plant sections were washed with three changes of sterile water inside a laminar flow hood. To prevent explant decay in vitro, bleached tissues from the end portion of the explant sections were removed with a sterile scalpel before in vitro establishment. Explants consisting of 1-2 cm long stem sections with a single axillary bud were established into 250 cm3 flasks containing 30 ml of the semisolid MS (Murashige & Skoog, 1962) medium with major salts, myoinositol (100 mg/L), sucrose (30,000 mg/L), thiamine HCl (0.4 mg/L), and Phytagel® (4,000 mg/L) (Sigma Co.). Flasks were covered with two layers of heavy-duty aluminum foil and sealed with Nescofilm®. Cultures were maintained at 20 °C, 12 h photoperiod (40 µmol m2 s-2), and monthly transferred to a fresh medium of the same formulation. After three consecutive subcultures, we recorded the number of explants alive, explants with newly formed shoots, contaminated explants, and phenolized media. Criolla explants, instead, were obtained from in vitro established plants maintained in similar conditions, as described for Martinera and Criolla 1 for one year with monthly transfers to a fresh medium. Original Criolla plants were obtained from field trials planted at the Universidad de Córdoba Experimental Field.
Shoot multiplication
To determine the best conditions for shoot multiplication from axillary meristems, clusters comprised of three shoots isolated from in vitro established Criolla, Criolla 1, and Martinera plants were transferred into 200 cm3 borosilicate flasks containing 30 ml of the semisolid MS medium with major salts and supplemented with myoinositol (100 mg/L), sucrose (30,000 mg/L), thiamine HCl (0.4 mg/L), and agar (8,000 mg/L) (Sigma Co.). Media were independently supplied at five benzylaminopurine (BAP) concentrations (0.0, 0.5, 1.0, 1.5, and 2.0 mg/L). Cultures were covered, sealed, and stored as indicated for the establishment stage.
The experiment consisted of a two-way factorial design (three cultivars × five BAP levels) with 15 treatments and five replicates; 75 experimental units (vessels) were distributed with a completely randomized design. Every 20 days, cultures were added with 5 ml of a fresh liquid medium of the same formulation, according to the respective treatment, and stored as indicated in the establishment stage. After medium addition for the fourth time (120 days in culture), we registered the number of new shoots per explant and the average shoot length (in cm from the base to the leaf tip of ten shoots from each treatment). Data were analyzed using an ANOVA based on the model (1), where μ was the general mean, Ƭi was the effect of the cultivar, βj was the effect of the BAP level, and Ɛiik was the experimental error. Means were separated with Tukey’s test (α = 0.05).
In vitro rooting
To evaluate rooting conditions, proliferated shoots (three-shoot clusters) from Criolla, Criolla 1, and Martinera were transferred into 200 cc borosilicate flasks filled with 30 ml of a semisolid MS major salts medium with myoinositol (100 mg/L), sucrose (30,000 mg/L), thiamine HCl (0.4 mg/L), and agar (8,000 mg/L) (Sigma Co.) and independently supplemented with five concentrations (0.0, 0.5, 1.0, 1.5 and 2.0 mg/L) of NAA. Light, temperature, and photoperiod conditions for cultures were similar, as indicated for the establishment and multiplication stages. After four weeks in the culture, we registered the number of cultures with roots, the number of roots per culture, and the average root length per culture. Collected data were analyzed with ANOVA based on (2), where μ was the general mean, Ƭi was the effect of the cultivar, βj was the effect of the NAA level, and Ɛiik was the experimental error. Means were separated with Tukey’s test (α = 0.05).
In vitro rooting stage is the most expensive stage of the micropropagation protocol due to auxin costs, labor, culture medium quantities, and reagents. Additionally, transfer from multiplication to rooting stage increases contamination risk, tissue stress, and culture time. The direct transfer of shoots from the multiplication stage to ex vitro adaptation reduces costs and time while maintaining plant survival rates and plant quality. Performance of both rooted and non-rooted shoots when transferred to ex vitro conditions proved that in vitro rooting is unnecessary for micropropagated plants to adapt to ex vitro conditions, which indicates that ex vitro transfer from multiplication stage can be performed with no implications for plant survival. Instead, time for plant production can be shortened and costs reduced.
The present protocol for micropropagation of arrow cane Criolla, Criolla 1, and Martinera using a double-phase medium and shoot transfer directly from the multiplication stage to ex vitro adaptation allows mass production of high-quality plants from few in vitro established explants in a relatively short time.
Acclimatization
In vitro rooted plants and unrooted shoots from each cultivar’s multiplication stage were transferred to ex vitro conditions to evaluate the plant survival rate. Micropropagated plants were extracted from the flasks, washed with distilled water to remove the medium, and placed in a 72-plug tray filled with peat (Pindstrup®) as substrate. A single plant was placed in every plug, sprayed with distilled water, and the tray covered with a transparent plastic cover. Trays were placed in a shade house (50% light) and irrigated every four hours using fog irrigation. After three days, covers were removed, and irrigation frequency was changed to one every eight hours. After 40 days, we recorded the number of plants that survived.
Results and discussion
In vitro establishment
Explant survival under in vitro conditions was affected by microbial contamination, low adaptation, and the disinfecting agent concentration. Out of 120 established explants from each cultivar, only five (4.1%) from Criolla 1 and seven (5.8%) from Martinera survived in vitro conditions, showing shoot growth from the axillary meristem. These explants were selected for further work (table 1, figure 1a). During the first two weeks, selected explants showed phenolic leaking, tissue blackening, and decay, which forced us to transfer them to a fresh medium every three days for two weeks to avoid explant death (figure 1b). New shoot formation was observed in few explants after five weeks (figure 1c). After 12-15 weeks, phenolic emission stopped, new emerging shoots and leaves shortened in size, organs went green, and flushing new shoots showed a radial growth pattern.
Table 1. In vitro establishment of arrow cane (G. sagittatum) cv. Criolla 1 and Martinera explants

Source: Elaborated by the authors

Photos: Claudia López Díaz
Figure 1. In vitro establishment process of arrow cane (G. sagittatum) explants in a semisolid MS (Murashige & Skoog, 1962) major salts medium: a. One week after establishment; b. Two weeks after establishment; c. Four weeks after establishment; d. Eight weeks after establishment.
Shoot multiplication
In vitro multiplied plants showed a radial growth pattern from an inside center point to the periphery with no evidence of adventitious shoot growth (figure 2).

Photos: Claudia López Díaz
Figure 2. Arrow cane shoot growth and multiplication from explants with pre-existing meristems cultured in a double-phase MS (Murashige & Skoog, 1962) medium supplied with 0.5 mg /L BAP after six- (left) and eight- (fight) week culturing in a multiplication medium.
ANOVA allowed detecting that cultivar, BAP levels, and the interaction between both statistically (Pr < 0.05) influenced new shoot formation. The Tukey’s test showed the highest rates of new shoot formation in Criolla (28.2), Martinera (21.2), and Criolla 1 (10) explants (table 2) when explants were cultured in a medium with 0.5 mg/L BAP, although, for Criolla 1 there were no statistical differences among shoots cultured in the presence or absence of BAP. The lowest multiplication rates occurred for all cultivars when explants were cultured in a medium without BAP supply. BAP level above > 0.5 mg/L drastically reduced new shoot formation (table 2, figure 3).
Table 2. BAP effect on in vitro shoot multiplication (standard deviation) and shoot length in cm (standard deviation) of arrow cane (G. sagittatum) cv. Criolla, Criolla 1, and Martinera

Source: Elaborated by the authors

Photo: Claudia Lopez Diaz
Figure 3. Arrow cane (G. sagittatum) shoot multiplication from explant with pre-existing meristems of Criolla (left), Criolla 1 (center), and Martinera (right) after four-week culturing in a double phase MS medium supplied with 0.5 mg/L BAP.
For shoot length, ANOVA allowed detecting statistical differences (Pr < 0.05) among all treatments. The longest shoots (10.8 cm) grew from Criolla shoots cultured with 0.5 mg/L BAP; however, it was not statistically different from Criolla 1 and Martinera shoots cultured at the same BAP level. Collected data showed a positive correlation (r = 0.78) between shoot length and BAP level in the medium (figure 3, table 2).
In vitro rooting
Cultures from all cultivars developed roots; therefore 100% rooting was observed for all treatments; however, ANOVA allowed detecting statistical differences (Pr < 0.05) among treatments due to cultivars, NAA levels, and the interaction between the two factors. Shoots cultured in NAA supplied media developed a higher number of roots than those cultured in the absence of NAA. The number of adventitious roots per explant was higher for most cultivars when the medium was supplied with 1.0 mg/L NAA; however, NAA level > 1.0 mg/L reduced the number of adventitious roots (table 3).
Table 3. In vitro adventitious root formation (standard deviation) and root length in cm (standard deviation) of cultures of arrow cane (G. sagittatum) cv. Criolla, Criolla 1, and Martinera in an NAA supplied medium

Source: Elaborated by the authors

Photos: Claudia López Díaz
Figure 4. Rooting of in vitro grown arrow cane (G. sagittatum) cv. Criolla (left), Criolla 1 (center), and Martinera (right) shoots after four-week culturing in an MS medium supplied with 1.0 mg/L NAA
The length adventitious roots were statistically affected by the NAA supply in the medium (Pr < 0.05). The Tukey’s test showed that the longest roots for Criolla 1 (6 cm), Martinera (3.8 cm), and Criolla (3.66 cm) occurred in shoots cultured in a medium without the NAA supply; in contrast, roots always shortened when explants were cultured in an NAA supplied media. Roots cultured in NAA were proportionally shortened with increased NAA concentration (figure 4, table 3).
Acclimatization
In vitro rooted plants from all cultivars and micropropagated shoots from the multiplication stage showed complete (100%) survival and full adaptation when transferred to ex vitro conditions (figure 5). Four weeks after transferring, plants were healthy, green-colored, and actively growing with no dead tissues, necrotic areas, or adventitious growth (figure 5).

Photos: Claudia López Díaz
Figure 5. Ex vitro adaptation of arrow cane (G. sagittatum) cv. Criolla, Criolla 1, and Martinera micropropagated plants.
Micropropagation is a clonal plant propagation technique that allows producing new plants from small size propagules (explants) under aseptic conditions, with controlled environmental conditions and heterotrophic growth, among other characteristics (Choudhary et al., 2015; Lodha et al., 2015). Micropropagation from explants with pre-existing meristems is one of the most used micropropagation methods in commercial plant material production because of genetic and phenotypic stability and high multiplication rates for most plant species (Choudhary et al., 2015; Hassan, 2017).
Micropropagation from pre-existing meristems is based on cytokinin addition to the culture media to disrupt apical dominance and induce repetitive axillary shoot elongation (Quiroz et al., 2017; Yavuz, 2016). Data obtained from the present research showed that, regardless of genotype, shoot multiplication od arrow cane in a double-phase system with 0.5 mg/L BAP statistically increased in Criolla and Martinera compared to those cultured in the absence of BAP. Previous reports on arrow cane micropropagation using semisolid system media are consistent with increased BAP effects on shoot multiplication from pre-existing meristems (Pastrana & Suárez, 2009; Suárez et al., 2009).
Criolla 1 showed the lowest multiplication rate (ten new shoots per explant) among all three cultivars at this BAP concentration; however, even for this cultivar, the observed multiplication rate, with a four- week frequency for subcultures, can become an efficient plant propagation method. Besides, since the shoot multiplication stage employed a double-phase medium, some other factors must be considered advantageous for this protocol, such as lower costs in gelling agents, less labor, reduced culture contamination, and lower plant stress. This fact may result in a more efficient micropropagation protocol for arrow cane plants (López & Suárez, 2018).
One of the purposes of shoot multiplication from pre-existing meristems is to obtain new shoots that can efficiently root and withstand ex vitro conditions for field adaptation (Lodha, 2015; Quiroz et al., 2017; Senapati, 2015). Shoot size is critical for energy reserve storage for adventitious root formation and new organ development when transferred to ex vitro conditions (Kane, 1996; Suárez Padrón, 2020). Multiplied arrow cane shoots in a semisolid conventional medium system with 0.5 mg/L BAP averaged 3 cm long (Pastrana & Suárez, 2009), while in the present research, shoots with the same BAP supply were three-fold larger (9 cm). Similar results were observed when comparing semisolid against double- phase cultured shoots of pineapple Ananas comosus L. (Bromeliaceae) (Scherwinski-Pereira et al., 2012) and arrow cane -G. sagittatum (López & Suárez, 2018; Suárez-Padrón et al., 2020). Increased shoot size is associated with more energy reserve accumulation, resulting in more chances of survival when transferred to ex vitro conditions and better plant performance during the initial phase of field growth and development (Almeida do Vale et al., 2019; Espinosa-Reyes et al., 2019).
The root is the organ that allows water and nutrient uptake and stabilizes the plant in the substrate (Quiroz et al., 2017; Senapati, 2015). Under in vitro conditions, plant medium and the environment prevent plant tissue from dehydration and favor nutrient uptake without root formation. However, for ex vitro conditions, in vitro rooting provides the mechanisms for normal growth and development of the plant in open field conditions (Choudhary et al., 2017; Zakavi et al., 2016). In vitro adventitious root formation is usually promoted by auxin supply in the culture medium (Ozdemir et al., 2014; Resende et al., 2016). The present research showed that NAA supply in the medium statistically increased the induction of adventitious root formation on micropropagated arrow cane shoots for all cultivars. Shoots cultured in NAA supplied medium formed three times more roots than those cultured in the absence of NAA; the response even increased when compared to previous arrow cane in vitro rooting studies (Pastrana & Suárez, 2009; Suárez et al., 2009).
Good quality in vitro rooting is not only the formation and proliferation of adventitious root but also root characteristics such as length and width (Benavides et al., 2016; Rodríguez et al., 2015; Zakavi et al., 2016). During the transfer to ex vitro conditions, shoots with shorter roots are easy to handle and do not require trimming, which produces stress and open wounds (Kane, 1996). Roots formed in NAA- supplied media were 50% shorter than those grown in NAA-deprived media, reducing root length as NAA levels increased for all cultivars, a pattern that seems to be similar for most in vitro cultivated plant species.
Stage IV of micropropagation is intended to give micropropagated plants the optimum environmental conditions to withstand transition from a heterotrophic (in vitro) to an autotrophic (ex vitro) growth model (Ozdemir et al., 2014; White et al., 2016). Low photosynthetic competence is one of the most severe limiting factors for in vitro conventionally cultivated plants to adapt to external field conditions. In vitro cultures are not actively photosynthetic since light intensity is not able to activate chloroplast function in normal in vitro conditions; therefore, sucrose must be added to the culture medium as an energy supply (Seon et al., 2000; Tisarum et al., 2018). Chloroplast dysfunction remains when plants are transferred to ex vitro conditions, and new organ formation, specifically leaves, is necessary to activate photosynthesis and autotrophic nutrition. Since no carbohydrate or other energy source is being produced, the energy required for new organ formation comes from accumulated reserves during in vitro growth (Lata et al., 2016; Nunes et al., 2016). The present study showed that double-phase-cultured shoots were three-fold larger than those micropropagated in conventional semisolid medium systems reported in other studies (Pastrana & Suárez, 2009; Suárez Padrón, 2020), increasing their ability for new organ formation and capacity to perform better during the in vitro.ex vitro transition.
A second limiting factor for in vitro grown plants transferred to ex vitro is rapid and severe tissue dehydration (Kumari et al., 2017; Mozafari et al., 2015). In vitro plants are maintained in a water- saturated closed atmosphere and established in a cultured medium with > 90% water. This fully humid environment prevents activation of mechanisms such as stomata closure and cuticle accumulation, intended to avoid dehydration. Since in vitro leaf structure is prone to rapid dehydration when transferred to ex vitro, plants must be placed in an environment with high water saturation (irrigation fog systems) and low light intensity (shade house) to avoid plant loss (Bukhari et al., 2016; Zakavi et al., 2016). In the present research, regardless of cultivar and stage of micropropagation (shoot multiplication or in vitro rooting), cultures transferred to ex vitro conditions fully adapted and survived (100% survival), as reported in previous studies (Pastrana & Suárez, 2009; Suárez et al., 2009).
Conclusions
Less than 10% of Criolla 1 and Martinera adapted to in vitro conditions. A 0.5 mg/L BAP supply in the medium statistically increased shoot multiplication rates for Criolla and Martinera. NAA supply in the medium increased adventitious root formation and reduced root length for all cultivar shoots; however, it did not affect rooting percentages. Micropropagated shoots for all cultivars showed complete (100%) adaptation and survival when transferred to ex vitro conditions, whether in vitro rooted or not.











 texto em
texto em