Introduction
Respiratory infections are significant causes of death in goats. Mycoplasma capricolum subsp. Capripneumoniae (Mccp) is the causative agent of the classical form of contagious caprine pleuropneumonia (CCPP), one of the most serious diseases in goats with high morbidity and mortality, causing economic losses in the goat industry1. As the disease is readily contagious, a short period of contact is enough for successful transmission and no evidence of indirect contact has been shown. The typical signs of CCPP include pulmonary consolidation, pleurisy and pleuropneumonia2. The exact geographical distribution of this disease is unknown by OIE, but it is indicated that CCPP is widely distributed in Africa and Asia3. Gathering information about the epidemiology of CCPP is useful for diagnosis, treatment and control strategies that have been used to improve knowledge about the distribution of CCPP.
Mannheimia haemolytica causes bronchopneumonia and affects sheep and goats. This opportunistic pathogen is found in the upper respiratory system of healthy goats, but stress induced by some situation like heat, overcrowding and transport make the bacteria cause pneumonia4. It can cause high economic losses due to treatment costs, slowdown in weight gain and decrease of meat and milk production5.
The present study was designed to detect two major causative agents of pleuropneumonia, Mccp and M. haemolytica, in grossly suspected goat’s lungs in Shiraz abattoir using the histopathological and molecular method.
Materials and Methods
Sample collection
50 samples of suspected lungs to pneumonia such as pulmonary consolidation and red discoloration and 10 healthy samples were collected during six months in Shiraz abattoir in southern Iran. The samples were immediately put in sterile plastic bags, placed in cooling boxes and were transported to the microbiology laboratory. Samples of both suspected and healthy lungs were considered for both tissue staining procedure and DNA extraction.
Histopathological examinations
All tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm in thickness and stained with hematoxylin and eosin (H&E).
DNA extraction
Briefly, one gram of each lung was separately homogenized in 1 mL distilled water and centrifuged (13000 g, 30 sec). Supernatant was removed and 1.5 mL distilled water was added to the tissue pellet, resuspended and centrifuged (13,000 g, 30 sec). This step was repeated twice for washing the tissues. An amount of 500 μL of lysis buffer (Tris-HCL, EDTA, 0.2% Tween; Sinaclon, Tehran, Iran) and 50 μL proteinase K (Sinaclon) was added and incubated overnight at 37 ˚C. The enzyme digested samples were mixed with 500 μL of phenol:chloroform:isoamylalcohol (25:24:1) for 15 min. Then, the mixture was centrifuged (10000 g, 10 min). The DNA was purified from the supernatant by adding equal volume of phenol: chloroform: isoamylalcohol (25:24:1) and was centrifuged at 10000 g for 1 min. The DNA was precipitated from the aqueous phase by addition of 2.5 volume of absolute etanol and incubated at -20 ˚C for 1 hr. The resultant DNA pellet was washed with 70% ethanol twice, dried and resuspended in 50 μL distilled water. Quality of extracted DNA was checked by Thermo Scientific NanoDrop (USA).
PCR assay for Mccp
The primer for Mccp was (5′ -ATCATTTTTAATCCCTTCAAG-3′, 5′ -TACTATGAGTAATTATAATATATGCAA-3′). PCR was performed in a Mini-MJ Bio Rad system. The specific amplification was performed in a 50 µl -final volume obtained by mixing 34 µl of distilled water, 0.5 µl of dNTP (each at 0.20 mM), 3 µl of MgCl2 (1.5 mM), 0.5 µl of 10U Taq Buffer (Sinaclon, Iran), 1 µl of each primer (0.4 mM of Mccp-spe-F: 50 -ATCATTTTTAATCCCTTCAAG-30 and Mccp-spe-R, 5’-TACTATGAGTAATTATAATATATGCAA-30 ), 0.5µl Taq DNA polymerase (5.00 U µL-1) and 5 µl of the sample. PCR conditions consisted of an initial denaturation step of 2 min at 94 ºC, followed by 35 cycles of 30 s at 94 ºC, 15 s at 47 ºC and 15 s at 72 ºC and a final extension step of 5 min at 72 ºC6. Negative and positive bacterial DNA controls were included for each PCR. The PCR products were subjected to electrophoresis in 1.5% agarose gel containing Gel Red (2µL per 50 gel) and the gel visualized under UV light.
PCR assay for M. haemolytica
The primer for M. haemolytica was (5′-GCAGGAGGTGATTATTAAAGTGG-3′, 5′ -CAGCAGTTATTGTCATACCTGAAC-3′). PCR condition and thermal cycle was carried out according to previously described by Alexander et al4.
Results
Gross and histopathological findings
Pulmonary consolidation in anterior and posterior lobes, red discoloration and occasionally pleural involvement, lung surfaces covered with a fibrin layer, were macroscopic lesions of suspected lungs. Histopathological evaluation revealed the most prevalent pneumonia was bronchointerstitial pneumonia, which observed in 40% (20 samples) of suspected simples with lesions including, peribronchiolitis, bronchiolitis, lymphocytic prevascular cuffing (PVC), interlobular septa and alveolar walls thickening due to infiltration of mononuclear cells and connective tissue and infiltration of neutrophils in bronchiolar and alveolar lumina (Figure 1a). Interstitial pneumonia was observed in 34% of suspected lungs with microscopic lesions including BALT, lymphocytic PVC, peribronchiolitis and thickening of alveolar walls because of infiltration of mononuclear cells (Figure 1b). The presence of mononuclear cells and eosinophils with Muellerius was observed in one sample. Two suspected lungs also showed type II pneumonocyte hyperplasia. Histopathological findings of samples with fibrinopurulent bronchopneumonia (10%) contained fibrin and numerous neutrophils infiltration in the lumina and thickening of interlobular septa due to fibrin deposition (Figure 1c). In addition, fibrinous thrombi represented in the lymphatic vessels of septa in some cases. 12% of suspected lungs had purulent bronchopneumonia with major lesion of neutrophils infiltration in the alveolar spaces (Figure 1d). Chronic pneumonia in 4% of samples with severe infiltration of mononuclear cells and connective tissue, lymphocytic PVC, BALT, peribronchiolitis and abundant alveolar macrophages was observed. There were no microscopic lesions in healthy lung samples.
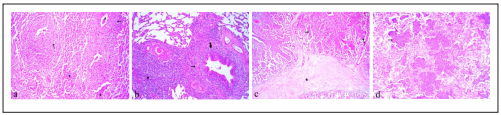
Figure 1 Histopathological section of lung tissues. a) Bronchointerstitial pneumonia with infiltration of neutrophils in bronchiolar and alveolar lumina (*), peribronchiolitis (arrow) and alveolar walls thickening. b) Interstitial pneumonia with peribronchiolitis (thin arrow), bronchiolitis (thick arrow) and severe infiltration of mononuclear cells (*). c) Fibrinopurulent bronchopneumonia with neutrophils infiltration in the lumina (arrow) and fibrin deposition in the interlobular septa (*). d) Purulent bronchopneumonia with numerous neutrophils infiltration in alveolar spaces (arrow). H&E, ×180.
PCR results
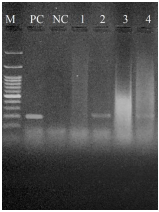
Although, the expected band size PCR for Mccp was 316 bp, the PCR showed no Mccp positive sample in all samples, neither suspected CCPP lungs nor healthy ones (Figure 2). The M. haemolytica PCR with 206 bp band size was observed in 14% (7 samples) of suspected samples (Figure 3) and no band in healthy lungs. In the 7 positive samples with M. haemolytica, lesions consisted of fibrinopurulent bronchopneumonia (four cases), bronchointerstitial pneumonia (two cases) and interstitial pneumonia (one case).
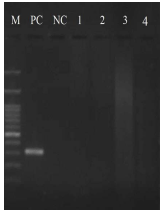
Figure 2 Electrophoretic analysis (1.5% agarose gel) of DNA amplified fragments for Mccp. M: DNA marker 100 bp (Sinaclon). PC: positive control; NC: negative control; Lanes 1-4: lung samples.
Discussion
Based on the present findings, the most prevalent pneumonia was bronchointerstitial pneumonia and interstitial pneumonia with major lesions including thickening of interlobular septa and alveolar walls due to infiltration of mononuclear cells, peribronchiolitis and lymphocytic PVC. In addition to mentioned lesions, infiltration of neutrophils in the lumina was observed in bronchointerstitial pneumonia. Histopathological lesions of CCPP have been reported, including thickening of interlobular septa, thickening of alveolar walls and lymphocytic PVC7. Mondal et al. reported the observation of interlobular septa thickening, lymphocytic PVC, fibrinous exudates, infiltration of neutrophils and vascular thrombosis, which are the same with observed lesions of fibrinopurulent pneumonia in this study8. In an experimental study, Gutierrez et al. reported lesions more in the form of interstitial pneumonia9.
Wesonga et al.10 experimentally inoculated 30 goats with Mccp and observed symptoms and lesions of CCPP, but no positive culture of bacteria was found. Even positive samples in immunohistochemistry were negative by molecular method that is in agreement with our findings.
In Ethiopia, Eshetu et al. reported 31% for the prevalence of CCPP by CFT. Also, they reported that gross and histopathological lesions of the lungs were indicative for pleuropneumonia caused by Mccp11. El-Deeb et al. confirmed Mycoplasma capricolum subsp. capripneumoniae in 55 isolates by PCR, which had showed fibrinous pleuropneumonia12. Awan et al. detected 17.65% positive samples for Mccp by PCR along with fibrinous pleuropneumonia and pleurisy in postmortem examination13. Other study in Turkey revealed 37.5% (12/32) CCPP in lung samples taken from goats14. In spite of some evidence of Mccp in mentioned countries, no positive case was detected in this abattoir study. Therefore, it may be needed to perform further studies through more samples and other techniques such as culture to confirm the lack of Mccp in our area.
In the present study, the most observed pneumonia in M. haemolytica positive samples by PCR was fibrinopurulent bronchopneumonia. This is relatively consistent with findings of previousstudies. Yener et al. represented 15 out of 19 M.haemolytica immunopositive pneumonic lungs were fibrinous bronchopneumonia15. Also, the study performed by Rawat et al. Showed fibrinous bronchopneumonia in pneumonic pasteurellosis16.
In conclusion, it seems that goats referring to Shiraz abattoir are free of Mccp and infected with M. haemolytica with the prevalence of 14%. As regards there is no epidemiological information on CCPP in our country, serological tests can be used to evaluate the overall distribution of the disease. In addition, sampling and molecular testing at the level of suspected herds to CCPP can be useful.