Introduction
Streptococcus constellatus is formally recognized within the Streptococcus anginosus group, which includes two other species, S. intermedius and S. anginosus1. This group frequently includes α-hemolytic Streptococci, although they may display β-hemolysis or appear as non-haemolytic2. It is important to mention that throughout history this group of bacteria has had a confusing and changing classification. They were previously included, as members of a group called Streptococcus milleri1,3. Even though this term is still used in the world medical journals literature, the anginosus group, proposed by Kawamura in 1995, is currently preferred, eliminating much of the confusion in the nomenclature4.
The microorganisms of the group Streptococcus anginosus are considered habitual microbiota of the oral cavity, throat, gastrointestinal and genitourinary tract; but they have been associated with serious contiguous or distant purulent infections when mucosal damage occurs especially in immunocompromised patients4. This type of compromise corresponds to one of the group’s most notable features, which confers it importance from the clinical point of view5. In this article, we describe the case of an immunocompetent patient with an unusual S. constellatus infection with multiorgan involvement.
Case report
A 46-year-old male, construction worker, presented to the emergency department reporting that he had a moderate intensity epigastric pain during 10 days. The patient also reported fever, fatigue, hyporexia, and diaphoresis predominantly at night. Three days before his admission, he presented melena feces. He had no other medical conditions and no prior surgeries. Tachycardia (HR=122 bpm) and hypotension (AP=87/55 mmHg) were evident on physical examination, in addition to dry, pale mucosa, poor dental hygiene and pain on abdominal palpation.
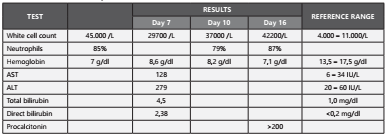
Laboratory findings at admission showed leukocytosis (45,000/mcL), neutrophils 85%, hemoglobin 7 gr/dl, bandemia (6%), and negative ELISA HIV test. An upper gastrointestinal tract endoscopy revealed a Murakami IV gastric ulcer. His initial treatment was sclerotherapy and transfusion of a globular concentrate.
Initial blood cultures were collected; and empirical treatment was started with ceftriaxone 2gr daily. On the fifth day, the initial blood cultures were reported as negative for this reason he completed 7 days of treatment with antibiotics; at the same time liver tests were documented with altered results.
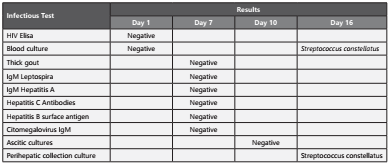
Thick drop, leptospira IgM, hepatitis A IgM, hepatitis C antibodies, hepatitis B surface antigen, and CMV IgM tests were taken, and they were reported as negative.
After 10 days of hospitalization the patient persisted febrile, and developed a lower extremity edema and ascites. An abdominal ultrasound, showed hypoechoic nodular images in liver segments IV and VIII. An abdominal CT scan, reported poorly defined focal liver lesions, portal vein thrombosis and intra-abdominal free fluid, an evacuation paracentesis of 2.5 liters of a slightly turbid fluid was performed; and it showed 4,712 leukocytes/mL, 70% neutrophils, 590 erythrocytes/mL, 68 mg/dL glucose, 2.8gr/dL proteins, and Gram stain and bacterial cultures incubated for 5 days were negative. The doppler ultrasound of the portal system confirmed portal vein thrombosis and anticoagulation was started with enoxaparin.
On day 12 of hospitalization, the patient continued with signs of inflammatory response, and a new cycle of empirical antibiotic therapy was initiated with meropenem 1 g intravenous solution every 8 hours for 7 days with general improvement. Subsequently, the patient presented fever again and abdominal pain in the right hypochondrium, with new negative blood cultures. The febrile episodes increased in frequency; and the patient persisted with leukocytosis, neutrophilia and procalcitonin higher than 200 ng/ml, Table 1 and Table 2 summarizes the results of the laboratory tests described in the case. The blood cultures were repeated, and they reported gram positive cocci which were typified as Streptococcus constellatus ssp. constellatus susceptible to penicillin (MIC <0.12 ug/mL) (Figure 1). Management was started with crystalline penicillin 30 million units daily by continuous intravenous infusion and intravenous moxifloxacin 400 mg daily.
An abdominal ultrasound was performed again; it showed an echogenic right subphrenic and perihepatic collection of 6x3cm, focal lesions of segment IV and VIII of the liver and a pelvic collection, anterior and superior to the bladder, of 9.1x8, 4x11cm. A CT guided percutaneous drainage was performed (Figure 2), obtaining 800cc of foul-smelling purulent material at subphrenic and pelvic level. A sample was sent for culture obtaining Streptococcus constellatus ssp constellatus (Figure 3). Drainage catheters were left which constant production.
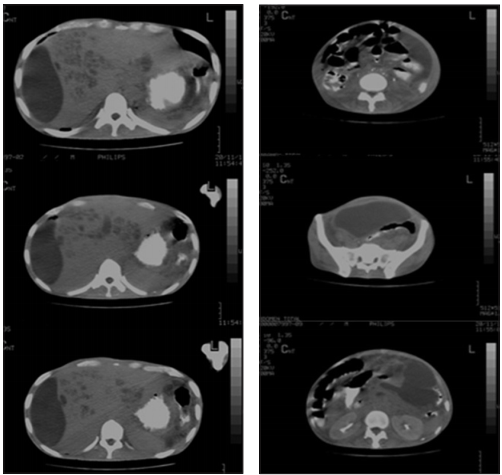
Figure 2a Subphrenic collection. Double contrast abdominal CT. Figure 2b. Pelvic collection. Double contrast abdominal CT.

Figure 3 Antibiogram of Streptococcus constellatus isolates in sample of purulent material at subphrenic and pelvic level.
It was decided to perform a surgery on the patient, and multiple foci were found in the intestinal loops. After surgical lavage, the patient was transferred to the ICU for monitoring, but he presented multi-organ failure and died. It is considered that the possible infectious origin was at the oral cavity level.
Discussion
As previously mentioned the members of this group, S. anginosus, are normally commensal pathogens found in the oropharyngeal, gastrointestinal and genitourinary microbiota5,6, but in the case of manipulations of the digestive or respiratory tract they can cause a great variety of abscesses in the abdominal cavity, lower respiratory tract, urogenital tract, orofacial region and in the skin3 as reported in different cases7,8, in contrast to this case, which had no history of surgical intervention and possible odontogenic foci. Binding of bacteria to extracellular matrix proteins is a common pathogenicity mechanism of streptococci that has been extensively studied and can also be observed in S. anginosus group. Streptococcus anginosus is typically found in the biofilm of dental plaque and adhesins play an important role in colonization of the oral cavity but it is also crucial for manifestation of clinical infections. A highly significant correlation between the binding to salivacoated hydroxyapatite and the ability to bind fibronectin were observed. This would explain the relationship between periodontal disease, poor dental hygiene and the development of invasive bacterial infections by members of this group9.
Other examples of identified Streptococcus anginosus group virulence factors include sialidase produced by S. intermedius, intermedilisyn - a cholesterol dependent cytolysin, which is probably involved in disease invasiveness as its level is high in abscesses and low in dental plaque, or chondroitin sulfate depolymerase and hyaluronidase. One of the most interesting discoveries regarding Streptococcus anginosus group virulence factors is the recent detection of genes encoding superantigens speC and speG and DNAses encoded by sdc and sdaD that were presumably transferred between species10.
In addition, metastatic abscesses in the brain, liver, spleen, bone, as well as endocarditis originating hematogenously have been described5. Furthermore, some studies have identified that members of this bacterial group have great etiological importance in the pulmonary exacerbations of patients with cystic fibrosis11. Recent analyses suggests that the number of infections caused by Streptococcus anginosus group is heavily underestimated. New data estimate the incidence of invasive Streptococcus anginosus group infections to be 8.65/100,000 population and 8.8/10,000 among hospitalized patients10.
It has been suggested that the different species of this group are associated with specific clinical syndromes. For example, S. constellatus is associated with thoracic infections, S. intermedius with central nervous system infections and S. anginosus with gastrointestinal and urogenital infections. However, a statistically significant correlation has not been demonstrated12,13. It is also known that patients with liver cirrhosis, cancer and diabetes mellitus are at greater risk of disseminated infections caused by the S. anginosus group12.
In this case, diagnosis was made with VITEK 2 automated system (bioMérieux, Marcy l’Etoile), which from an inoculum, from a previously purified culture, performs a series of biochemical tests that deliver highly sensitive and specific results14,15.
In regard to treatment of infections by S. anginosus group microorganisms, they are known to be susceptible to penicillin, and for this reason, taxonomic classification up to the species level is not routinely carried out13. Specifically in relation to S. constellatus infection, it is known that these microorganisms show susceptibility to β-lactam antibiotics, doxycycline, erythromycin, vancomycin, and intermediate susceptibility to clindamycin is also reported16. Despite its good response to antibiotic therapy, an increase in resistance to penicillin and clindamycin has been described in different studies17. In one study subgingival isolates of S. constellatus and S. intermedius in vitro were all or nearly all susceptible to amoxicillin, clindamycin, and azithromycin, only intermediate in susceptibility to ciprofloxacin, frequently resistant to doxycycline, and nearly all resistant to metronidazole. Subgingival S. constellatus and S. intermedius exhibited variable antibiotic susceptibility profiles, potentially complicating empiric selection of periodontitis antibiotic therapy in species-positive patients18. It is speculated that abscesses in sterile sites of the host organism are probably formed as a result of the high resistance of S. anginosus group sus group capsule is one of the better characterized virulence factors. Encapsulated strains are more virulent in the murine abscess model as the capsule affects phagocytosis and neutrophil killing. Possible molecular mechanisms involved in that process may also include the production of hydrogen sulfide from L -cysteine by L - cysteine desulfhydrase10.
In addition to parenteral antimicrobial therapy, the treatment of an abscess involves the drainage of purulent material. The sole medical therapy should be reserved for patients with multiple and/or small, well-defined lesions or when patients are at high risk of surgical complications12.
In this case, the S. constellatus strain had a susceptibility spectrum to penicillin (MIC <0.12 ug/mL) which allowed to define management with crystalline penicillin and moxifloxacin 400 mg due to infections diseases recommendation. Given the high rate of susceptibility to penicillin, this drug remains the first line of treatment19. In addition, intraabdominal infections are usually polymicrobial, studies have reported that the incidence of polymicrobial bacteremia was as high as 51%19, in one study liver abscesses were associated with polymicrobial infections in 50% of the cases20, Clarridge et al. reported that S. intermedius tends to be isolated as a sole pathogen, whereas S. anginosus and S. constellatus are associated with polymicrobial infections5. The results of one report pooling 4 wellcontrolled trials21, supports the asseveration that moxifloxacin is efficacious for the treatment of intra-abdominal infections caused by a broad range of anaerobic and aerobic bacteria, being coherent with the published SIS and IDSA guidelines, which recommend moxifloxacin for the treatment of mild-tomoderate community-acquired intra-abdominal infections21.
Based on the result of abdominal CT, a percutaneous drainage was performed in which the growth of the bacteria was evidenced. This along with the positive blood cultures allows to infer the capacity that this microorganism has to produce metastatic infections which must be properly identified and treated to avoid persistence of the infection.
Although typifying this group at the species level is not essential to start treatment, It is suggested that this taxonomic identification has prognostic importance, because of the loss of life in 30 days and the average length of hospital stay being significantly higher in S. intermedius infections than in patients infected with S. constellatus and S. anginosus11, with a general mortality rate of 16% found for all subspecies11. In this sense, prospective studies are necessary to verify whether infection by a specific species of the group is a prognostic factor of the severity of the infection and of the clinical outcome.
This patient illustrates an unusual infection with multiple intraabdominal abscesses, sepsis and septic shock with subsequent multiorgan failure by Streptococcus constellatus. It is also observed that despite having a trained team of doctors, the facilities provided by a regional hospital in terms of diagnostic methods and adequate treatment based on surgical drainage and susceptibility spectrum, the result was not what it was expected. This highlights the relevance of early diagnosis.