Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232On-line version ISSN 2357-3759
Caldasia vol.30 no.2 Bogotá July/Dec. 2008
MARISOL AMAYA-MÁRQUEZ
HARRINGTON WELLS
Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá D. C., Colombia. mamayam@unal.edu.co
Department of Biology, University of Tulsa, Tulsa, Oklahoma 74104, U.S.A. harrington-wells@utulsa.edu
ABSTRACT
Social complexity and models concerning central place foraging were tested with respect to learning predictions using the social honey bee (Apis mellifera ) and solitary blue orchard bee (Osmia lignaria) when given foraging problems. Both species were presented the same foraging problems, where 1) only reward molarity varied between flower morphs, and 2) only reward volume varied between flower morphs. Experiments utilized blue vs. white flower patches to standardize rewards in each experimental situation. Although honey bees learned faster than blue orchard bees when given a molarity difference reward problem, there was no significant difference in learning rate when presented a volume difference reward problem. Further, the rate at which blue orchard bees learned the volume difference problem was not significantly different from that with which honey bees learned about reward molarity differences. The results do not support the predictions of the social complexity theory, but do support those of the central place model.
Key words. Osmia lignaria, Apis mellifera, learning in solitary and social bees, foraging, social complexity model, central place foraging.
RESUMEN
Las predicciones sobre aprendizaje derivadas de los modelos de complejidad social y forrajeo central, fueron sometidas a prueba usando una especie de abeja social (Apis mellifera) y una especie de abeja solitaria (Osmia lignaria), a las cuales les fue ofrecida una tarea de aprendizaje en el contexto de forrajeo de néctar. Ambas especies fueron expuestas a las mismas condiciones de forrajeo, donde 1) se cambió únicamente la molaridad de la recompensa entre las formas florales, y 2) se cambió únicamente el volumen de la recompensa. Los experimentos se hicieron con parches de flores artificiales utilizando dos variedades de color floral (azul y blanco) para estandarizar las recompensas de néctar en cada situación experimental. Aunque las abejas sociales aprendieron más rápido que las solitarias cuando se les presentó un problema de diferencia de molaridad en la recompensa, no hubo una diferencia significativa con respecto al aprendizaje de diferencias en volumen de néctar. Adicionalmente, la tasa a la cual O. lignaria aprendió diferencias en el volumen de la recompensa, no difirió de la tasa a la cual A. melifera aprendió las diferencias de concentración del néctar. Los resultados no apoyan las predicciones de la teoría de la complejidad social, pero soportan las del modelo de forrajeo central.
Palabras clave. Osmia lignaria, Apis mellifera, aprendizaje en abejas solitarias y sociales, forrajeo, modelo complejidad social, forrajeo central.
INTRODUCTION
Animals exhibit a wide spectrum of social organizations ranging from solitary to highly eusocial species (Wilson 2000). Potential benefits afforded social organisms include foraging efficiency, defense against predators, thermoregulation and mate location (Alcock 2001; Brown 1986, 1988; Wells et al. 1990; Wells & Wells 1992). As social structure increases, an individual's role in the society becomes more specialized, even to the point where only a few individuals of the group reproduce (Wilson 1971). With socialization comes the ability to recognize individuals based on kin relationship and status within the group (von Frisch 1967). Also required of social species is that individuals within the society must work together for the common good, which requires odor, mechanical or visual communication of needs (Seeley 1985; Michener 1985; Wenner & Wells 1990). Thus, social complexity theory has posited that social individuals have more developed cognitive abilities than corresponding solitary species (Kamil 2004). That is, cognitive differences between social and solitary species derive from the contingencies of a social life, and are the basis for social complexity theory (Essock-Vitale & Seyfarth 1986; Cheney & Seyfarth 1990; Bond et al. 2003).
Bees are an excellent model system to test the ideas about social complexity and cognitive ability. As a taxon, they are diverse in terms of social organization (Michener 1969). Further, bees in diverse genera are able to recognize conspecifics and heterospecifics, and exhibit differential behaviors toward each (Wcislo 1997; Breed 1998; Keller & Ross 1998). Finally, there are solitary and eusocial species that utilize the same food resource base. In particular, there are many species that rely on nectar and pollen. Both solitary and social pollinator bees face the same challenges in foraging and provisioning brood cells. However, solitary and eusocial bee species differ in how they interact with kin, and solitary species do not have overlapping generations (Michener 1985; O'Toole & Raw 1991).
In contrast to social complexity theory is the idea that both solitary and social bees, in fact, do not differ in cognitive abilities because foraging for pollen and nectar, whether or not an individual is a member of a social group, requires the same skills in order to successfully compete with other species. Further, bees are central place foragers: they forage from a home base to which they return. Thus, foragers of both social and solitary bees require spatial cognition abilities that enable them to find food in an unpredictable environment and return repeatedly to their own nest. In fact, both social and solitary bees have been shown not only to do this, but also to be able to respond to the movement of their hive/nest from the original location (Gathmann & Tscharntke 2002).
Nest building by solitary bees as well as by eusocial bees, requires great energy expenditure. Solitary bees are burdened by the energy deficit accrued in building a nest using mud in cell construction (e.g. Chalicodoma spp., Osmia spp.), tunneling in wood (e.g. Xylocopa spp.), or digging soil in fossorial species (e.g. Dasypoda spp.); most bees prefer to use preemptied cavities, rather than to dig one (O'Toole & Raw 1991). In fact, suitable nest sites may well be more of a limiting factor than are flowers for many solitary bees, and so it is not surprising that many solitary bees reuse nests for several years (Hurd & Moure 1961; Garófalo et al. 1992; Gathmann & Tscharntke 2002; Kukuk 2002). Of course, the structural and time investment in the hive by honey bees is well known, and need not be discussed further (Seeley 1985). Because foraging from a central place appears to entail similar costs in both solitary and social bees, both social and solitary bees should have equal cognitive abilities associated with foraging (Menzel 2001). Further, flower memory seems to be linked to spatial memory in bees (Menzel 2001), and so it is not surprising that solitary as well as eusocial bees have good spatial memories (Gathmann & Tscharntke 2002). Thus, the central place forager model suggests that social and solitary bees should not differ in their cognitive abilities.
In this study honey bee (Apis mellifera) and blue orchard bee (Osmia lignaria) learning rates in a foraging context are compared when given the same problems. Honey bees are highly eusocial, and have a system of task specialization. On the other hand, blue orchard bees are solitary, and each female must reproduce, forage and build a nest. Both species are generalist foragers and visit a wide variety of flowering plant species. However, particular environments or seasons may restrict flower type diversity visited by either honey bees or blue orchard bees.
MATERIALS AND METHODS
Experiments were conducted using blue orchard bees (Osmia lignaria) and the honey bee (Apis mellifera). Blue orchard bees and honey bees were naïve for the experimental conditions. Each species was tested separately.
Experiments with Blue orchard bees were carried out outdoors between April and June 2005 in Tulsa, Oklahoma, inside a cubic net cage (2.5m x 2.5m x 2.5m). Bees were obtained commercially from Knox Cellar in their over-wintering stage, kept refrigerated (4 oC) until used in an experiment and confined to the cage once removed from the refrigerator. A straw containing over-wintering bees was placed into a hole in a wood block in the outdoor cage 2 days prior to the initiation of an experiment. Bees emerged serially from the straw, on average two bees per day. A wood block 14 x 12 x 24 cm with 72, 8mm holes, in a 6 by 12 Cartesian coordinate system was located inside the cage for nesting. Red begonias were offered as pollen-flowers and an artificial flower patch (design of Sanderson et al. 2006) was used to provide bees with nectar-flowers. An experiment was initiated by placing an artificial flower patch into the cage. Bees were individually marked for identification using enamel paint dots on the thorax or abdomen. A visit was recorded whenever a bee entered the corolla tube. The flower color sequence that each bee visited was recorded. Revisits to the same flower were not explicitly excluded, but flowers were refilled with the same quality and quantity of reward only after a forager had moved to another flower. Each experiment used a new set of bees.
Experiments with honey bees were conducted outdoors in Tulsa, Oklahoma between June and July 2005. The bees were baited to visit a Petri dish containing a sucrose solution 1.5 M clove scented 1µl/L. At the time of the experiment the feeder was replaced for an artificial flower patch. Bees were marked as indicated above for blue orchard bees. The flower color sequence of visits made by individual bees was recorded. Each experiment used a new set of bees.
Flowers
Artificial flower patches consisted of 36 flowers spaced 75mm apart in 6 rows and 6 columns of a Cartesian coordinate system on a brown pegboard. A flower consisted of a 28mm x 28mm Plexiglas square, 6mm thick, with a 8mm internal diameter (ID) 15mm deep Plexiglas tube that held the reward recessed in one corner. Each flower was mounted on a 90mm pedicel of 5mm doweling. Flowers were either blue or white. Blue and white flowers occurred in equal number and were randomly arranged as to color in a flower patch. Flower colors were created by painting the lower surface of each Plexiglas square with blue or white enamel paint, and wrapping the tube with translucent film so as to match the underside of the Plexiglas square (Testors™ paint Nos. 1208 blue, 1245 white; Roscolux film Nos. 63 blue, 100 white-frost).
Experiments
Experiments 1 and 2 varied reward sucrose molarity. Experiment 1 used blue orchard bees and experiment 2 used honey bees. These experiments had two treatments performed sequentially without breaks. Treatment I had all flowers offering 2ul of 1ul/L clove scented 1 M sucrose. Treatment II (training) had one flower color offering 2ul of 1ul/L clove scented 2M sucrose and the other flower color 2ul of 1ul/L clove scented 1M sucrose.
Experiments 3 and 4 varied reward volume. Experiment 3 used blue orchard bees and experiment 4 used honey bees. Treatment I was unchanged from that used in experiments 1 and 2, but Treatment II had one flower color offering 6ul of 1ul/L clove scented 1M sucrose, and the other flower color offering 2ul of 1ul/L clove scented 1M sucrose.
When honey bees were the experimental organism, approximately half of the bees tested had white flowers (and the other half blue flowers) with the greater caloric reward in Treatment II.
However, blue orchard bees previously have shown a distinct innate preference for blue flowers, and so were trained only with white flowers offering the greater caloric reward in Treatment II. As an experimental control when using bees (blue orchard and honey bee), experiments were repeated but with Treatment II rewards the same as Treatments I (i.e. there was no difference in rewards between flower colors).
Data Analysis
The ability to associate reward with color was examined by fitting data from each experiment separately to the learning rate model of Dukas & Real (1991): P = 1 – ce-dv following Dukas & Real (1991) where P = cumulative relative frequency of white flowers visited divided by the number of flowers visited, c = initial preference for blue flowers, d = learning factor, and v = number of flower visits. Model parameters were estimated using least-squares linear regression on log-transformed data (i.e. ln(1–P) = ln(c) –dv). An ANOVA was used to test for significance of each regression (JMP IN: Sall & Lehman 1996). An ANOVA was used to test for significant differences among the regression slopes, which is the learning factor d (following Sokal & Rolhf 1995).
RESULTS
Regression analyses were significant for Osmia when reward molarities were different (Expt. 1: F=49.80; df=1,13; P<0.0001), Apis when reward molarities were different (Expt. 2: F=1200.07; df=1,13; P<0.0001), Osmia with reward volume differences (Expt. 3: F=256.53; df=1,13; P<0.0001), and Apis with reward volume differences (Expt. 4: F=548.09; df=1,13; P<0.0001). Thus, the learning coefficient was significantly different than zero for both species when presented a reward volume or molarity foraging problem (Fig. 1).
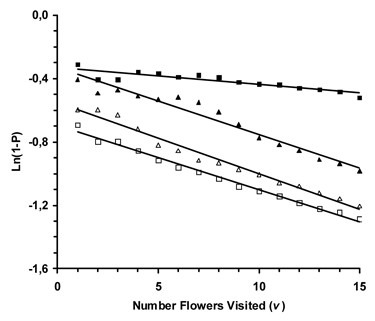
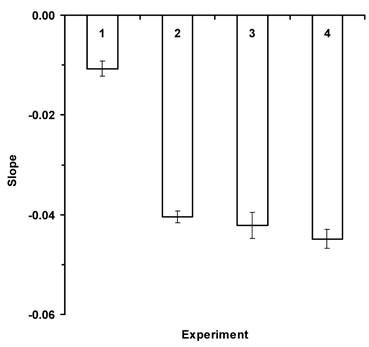
In addition, significant differences existed among the regression slopes, which are the learning coefficients (F=89.50; df=2,39; P<0.0001). However, when regression slopes with standard error are plotted (Fig. 2) it is apparent that those for Apis (reward molarity and volume) plus Osmia when reward volume varied do not differ (Expts. 2, 3 & 4). The learning coefficient when Osmia was presented the reward molarity problem (Expt. 1) was significantly less than in the other experiments. Results are based on the first 15 flowers visited in Treatment II for each bee (Osmia and Apis). Results are based on 546 flowers visited by 15 blue orchard bees for Experiment 1, 1847 flowers visited by 20 honey bees in Experiment 2, 657 flowers visited by 19 blue orchard bees in Experiment 3, and 1, 1208 flowers visited by 19 honey bees in Experiment 4.
DISCUSSION
Social and solitary bees exhibited behavioral plasticity when they exploited the experimental floral patches. Both bee species were sensitive to the caloric value of the flower reward, whether nectar quality or quantity, were varied. Bees of both species changed flower species preference during training (Treatment II). The results do not support the social complexity hypothesis (Kamil 2004) because differential rates of learning were not consistently associated with a particular bee species. That is, the solitary blue orchard bee learned the reward volume problem as fast as the social honey bee. Further, honey bee learning when there was a reward molarity problem was not faster than when either honey bees or blue orchard bees were given the reward volume problem. Thus, sociality is not the factor explaining the cognitive abilities exhibited by bees exploiting the floral patches. Our results are consistent with the predictions derived from central place foraging, supporting the idea that the cognitive system of bees involved in learning about flowers is linked to a more inclusive cognitive system related to spatial memory (Menzel 2001).
Cognitive similarities between solitary and social species of Hymenoptera have been reported outside the domain of flower exploitation. In context-dependent social interactions, it has been recognized that an individual female of a solitary bee species is able to recognize conspecifics and modify her behavior in accordance (Wcislo 1997); similar capacities are known from solitary wasp studies (Pfennig & Reeve 1989), and from individuals of social bee species (Michener and Smith 1987). These results suggest that intelligence in bees is derived from the ancestral solitary forms (West-Eberhard 1989, 2003).
A condition for sociality to evolve in Hymenoptera is the ability of ancestral solitary wasps and bees to recognize other individuals (Gadagkar 2001), and thus rely on mutual reciprocation to make group living an evolutionary stable strategy (ESS) (Cheney & Seyfarth 1982; Silk et al. 1999; Giraldeau & Caraco 2000). It may seem paradoxical to attribute an adaptive value to the ability to recognize other individuals in solitary species, but many solitary bee species are gregarious because nesting places are rare. In fact, nest sites are probably a more limiting resource than flowers for solitary bees (Hurd & Moure 1961; Gathmann & Tscharntke 2002). This leads to recurrent nest use by bees for several years by some species. Wcislo & Cane (1996: p. 257) have pointed out that "nesting of solitary bees in localized areas influences the intensity of interactions with enemies and competitors." Therefore, not only the distinction between solitary and social species based on life cycle, but also ecological associations are relevant when testing the social complexity hypothesis.
The differential learning rate shown by the blue orchard bee and honey bee to nectar quality and quantity may relate to water requirements. Water is a resource involved in thermoregulation of individual bees (Willmer 1991), as well as for the masonry used in orchard bee nest construction. The economic necessity of water for bees rarely has been taken into account in relation to foraging. However, bees sometimes derive water from nectar (Willmer 1986), and that may explain interspecific differences observed here. This idea is worth further study, and can be examined even within the same species by controlling for different environmental conditions (temperature/humidity). If the bees have a significant water need from nectar then preference for different nectar, types should be correlated with weather regimes.
ACKNOWLEDGEMENTS
To the National University of Colombia and University of Tulsa for the opportunity to do research. To Jorge Sebastian Figueroa for his insight on the behavior of the Blue Orchard Bee. To Dr. Peter Kevan and other two anonymous reviewers whose observations contributed to improve the manuscript.
LITERATURE CITED
1. ALCOCK, J. 2001. Animal Behavior. An Evolutionary Approach. Sinauer Associates, Inc. Sunderland, Massachusetts. 420-451 pp. [ Links ]
2. BOND, A.B., A.C. KAMIL & R.P. BALDA. 2003. Social complexity and transitive inference in corvids. Animal Behaviour 65:479-487. [ Links ]
3. BREED, M.D. 1998. Recognition pheromones of the honey bee. BioScience 48:463-470. [ Links ]
4. BROWN, C.R. 1986. Cliff swallow colonies as information centers. Science 234:83-85. [ Links ]
5. BROWN, C.R. 1988. Social foraging in cliff swallows: Local enhancement, risk sensitivity, competition and the avoidance of predators. Animal Behaviour 36:780-792. [ Links ]
6. CHENEY, D. & R.M. SEYFARTH. 1982. Recognition of individuals within and between groups of free-ranging Vervet monkeys. American Society of Zoologists 22:519-529. [ Links ]
7. CHENEY, D. & R.M. SEYFARTH. 1990. How Monkeys See the World: Inside the Mind of Another Species. University of Chicago Press, Chicago. [ Links ]
8. DUKAS, R. & L.A. REAL. 1991. Learning foraging tasks by bees: A comparison between social and solitary species. Animal Behaviour 42:269-276. [ Links ]
9. ESSOCK-VITALE, S. & R.M. SEYFARTH. 1986. Intelligence and social cognition. Pp. 452-461 in: Primate Societies. Smuts, B., D. L. Cheney, R. M. Seyfarth, R. W. Wrangham & T. T. Struhsaker (eds.) University of Chicago Press, Chicago. [ Links ]
10. FRISCH, K. VON 1967. The Dance Language and Orientation of Bees. Harvard University Press, Cambridge, Massachusetts. [ Links ]
11. GADAGKAR, R. 2001. The Social Biology of Ropalidia marginata. Harvard University Press, Cambridge, Massachusetts, London. [ Links ]
12. GARÓFALO, C.A., E. CAMILLO, M.J.O. CAMPOS & J.C. SERRANO. 1992. Nest re-use and comunal nesting in Microthurge corumbae (Hymenoptera, Megachilidae), with special reference to nest defense. Insectes Sociaux 39: 301-311. [ Links ]
13. GATHMANN, A. & T. TSCHARNTKE. 2002. Foraging ranges of solitary bees. Journal of Animal Ecology 71:757-765. [ Links ]
14. GIRALDEAU, L.A. & T. CARACO. 2000. Social Foraging Theory. Princeton University Press, Princeton, New Jersey. [ Links ]
15. HURD, P.D. & J.S. MOURE. 1961. Some notes to the genus Xylocopa Latreille (Hymenoptera: Aculeata). Journal of the Kansas Entomological Society 34:19-22. [ Links ]
16. KAMIL, A.C. 2004. Sociality and the evolution of intelligence. Trends in Cognitive Sciences 8:195-197. [ Links ]
17. KELLER, L. & K.G. ROSS. 1998. Selfish genes: A green beard in the red fire ant. Nature 394:573-575. [ Links ]
18. KUKUK, P.F. 2002. Nest Reuse in a Communal Halictine Bee, Lasioglossum (Chilalictus) hemichalceum (Hymenoptera: Halictidae). Journal of the Kansas Entomological Society 75: 3-7. [ Links ]
19. MENZEL, R. 2001. Behavioral and neural mechanisms of learning and memory as determinants of flower constancy. pp. 21-40 in: Cognitive Ecology of Pollination. Animal Behavior and Floral Evolution. Chittka, L. & J. D. Thomson (eds.) Cambridge University Press, Cambridge, UK. [ Links ]
20. MICHENER, C.D. 1969. Comparative social behavior of bees. Annual Review of Entomology 14:299-342. [ Links ]
21. MICHENER, C.D. 1985. From solitary to eusocial: Need there be a series of intervening species?. pp. 293-305 in: Experimental Behavioral Ecology and Sociobiology: in Memoriam Karl von Frisch 1886-1982. Hölldobler, B. & M. Lindauer (eds.). Erlbaum Associates. Hillsdale, New Jersey. [ Links ]
22. MICHENER, C.D. & B.H. SMITH. 1987. Kin recognition in primitively eusocial insects.p. 209-242 in: King Recognition in Animals. Fletcher, D. J. C. & C. D. Michener (eds.). John Wiley & Sons, Ltd., Chichester. [ Links ]
23. O'TOOLE, C. & A. RAW. 1991. Bees of the World. Facts on File, Inc., New York. [ Links ]
24. PFENNIG, D.W. & H.K. REEVE. 1989. Neighbor recognition and context-dependent aggression in a solitary wasp, Sphecius speciosus (Hymenoptera: Sphecidae). Ethology 80:1-18. [ Links ]
25. SALL, F. & A. LEHMAN. 1996. JMP IN. SAS Institute, Inc. Ducksberry Press, Belmont, California. [ Links ]
26. SANDERSON, C.E., B.S. OROZCO, P.S.M. HILL & H. WELLS. 2006. Honey bee (Apis mellifera ligustica) response to differences in handling time, rewards, and flower colours. Ethology 112:937-946. [ Links ]
27. SEELEY, T.D. 1985. Honey bee Ecology. A Study of Adaptation in Social Life. Princeton University Press, Princeton, New Jersey. [ Links ]
28. SILK, J.B., R.M. SEYFARTH & D.L. CHENEY. 1999. The structure of social relationships among female savanna baoons in Moremi reserve, Botswana. Behaviour 136:679-703. [ Links ]
29. SOKAL, R.R. & F.J. ROHLF. 1995. Biometry. W. H. Freeman and Company, New York. [ Links ]
30. WCISLO, W.T. 1997. Social interactions and behavioral context in a largely solitary bee, Lasioglossum (Dialictus) figueresi (Hymenoptera, Halictidae). Insectes Sociaux 44:199-208. [ Links ]
31. WCISLO, W.T. & J.H. CANE. 1996. Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annual Review of Entomology 41:257-287. [ Links ]
32. WELLS, H. & P.H. WELLS. 1992. The monarch butterfly: A review. Southern California Academy of Sciences 91:1-25. [ Links ]
33. WELLS, H., P.H. WELLS & P. COOK. 1990. The importance of monarch butterfly (Danaus plexippus) winter aggregations to successful reproduction. Journal of Theoretical Biology 147:115-131. [ Links ]
34. WENNER, A.M. & P.H. WELLS. 1990. Anatomy of a Controversy. Columbia University Press, New York. [ Links ]
35. WEST-EBERHARD, M. 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics 20:249-278. [ Links ]
36. WEST-EBERHARD, M. 2003. Developmental Plasticity and Evolution. Oxford University Press, Inc., New York. [ Links ]
37. WILLMER, P.G. 1986. Foraging patterns and water balance: Problems of optimization for a Xerophilic bee, Chalicodoma sicula. The Journal of Animal Ecology 55:941-962. [ Links ]
38. WILLMER, P.G. 1991. Constraints on foraging by solitary bees. pp. 131-148 in: The Behavior and Physiology of Bees. Goodman, L. J. & R. C. Fisher (eds.). CAB International, Wallingford, UK. [ Links ]
39. WILSON, E.O. 1971. The Insect Societies. The Belknap Press of Harvard University Press, Cambridge, Massachusetts. [ Links ]
40. WILSON, E.O. 2000. Sociobiology. The New Synthesis. Twenty-Fifth Anniversary Edition. The Belknap Press of Harvard University Press, Cambridge, Massachusetts. [ Links ]
Recibido: 18/10/2007
Aceptado: 14/05/2008














