Remark
| 1) Why was this study conducted? |
| Damage control resuscitation should be initiated as soon as possible after a trauma event to avoid metabolic decompensation and high mortality rates. The aim of this article is to describe the pre-hospital attention principles and our experience regarding Stop the Bleed initiative within Latin America. |
| 2) What were the most relevant results of the study? |
| The use of tourniquets is recommended as a measure to control bleeding. Fluid management should be initiated using low volume crystalloids keeping the permissive hypotension principle. Hypothermia must be management using warmed blankets or the administration of intravenous fluids warmed prior to infusion. However, these prehospital measures should not delay the transfer time of a patient from the scene to the hospital. |
| 3) What do these results contribute? |
| Prehospital damage control measures are the first steps in the control of bleeding and the initiation of hemostatic resuscitation in the traumatically injured patient. |
Introduction
Severe traumatic hemorrhage is the leading preventable cause of death among young adults 1,2. The early care of a trauma patient can be challenging, with a high mortality rate especially in the first two hours after injury, which makes the bleeding control a time-sensitive condition3,4. Damage control resuscitation (DCR) is an effort to understand the metabolic response to trauma and what measures can be implemented to improve this physiological deterioration. The concept of DCR evolved from the principles of damage control surgery 5. In 2001, phase 0 of damage control surgery was introduced, focusing on early damage control measures that can be taken in the prehospital setting 6. However, it is still an open debate which measures should be implemented, especially those involving bleeding control in the prehospital setting 7-10. This is why, the Trauma and Emergency Surgery Group (CTE) group from Cali, Colombia has developed a campaign in Colombia and other Latin American countries including Costa Rica and Ecuador, with the aim of teaching basic damage control techniques for health care provides or community personnel through the Stop the Bleed Course. The aim of this article is to state our position regarding prehospital care and damage control resuscitation, including our experience with the “Stop the Bleed” Campaign in Latin America.
This article is a consensus that synthesizes the experience earned during the past 30 years in trauma critical care management of the severely injured patient from the Trauma and Emergency Surgery Group (CTE) of Cali, Colombia which is made up of experts from the University Hospital Fundación Valle del Lili, the University Hospital del Valle "Evaristo García", the Universidad del Valle and Universidad Icesi, the Asociación Colombiana de Cirugia, the Pan-American Trauma Society and the collaboration of national and international specialists of the United States of America and Latin America.
Epidemiology
Trauma is one of the leading causes of death worldwide. According to the World Health Organization (WHO), 4.9 million people died in 2016 due to any type of trauma 11. Mortality rates were higher in low-income countries, mainly due to traffic road accidents. In Colombia, the Departamento Administrativo Nacional de Estadistica (DANE) 12 reported that the trauma is the main cause of death among young adults and the second leading cause of death in general population with a total of 34,216 deaths in 2007. 54% of all patients who died between 1998 and 2011, were considered to died due to preventable causes, the most common trauma mechanism was gunshot wounds, followed by stab wounds, and traffic road accidents 12,13.
The 50% of patients with severe hemorrhage do not receive timely healthcare and die before hospital admission. In 2014, 1848 trauma-related deaths were analyzed, finding that 305 cases were related to non-controlled hemorrhage. Uncontrolled hemorrhage was identified as the cause of death in 305 patients. 44.9% of these deaths were attributed to potentially preventable hemorrhage. 35.8% of these deaths occurred in the prehospital setting and 20.4% occurred within the first hour prior to admission. Trauma to a non-compressible body zone was present in 74.5% of these deaths, 21.6% occurred in the chest, 38.2% in the chest and abdomen, 15.7% in the abdomen alone and 24.5% in another area 14.
Pathophysiology
Damage control resuscitation in a prehospital setting focuses on the aggressive prevention and treatment of the lethal triad of hypothermia, acidosis, and coagulopathy described by Moore et al. in 1996 15, and recently expanded by Ditzel et al. to lethal diamond with the inclusion of hypocalcemia 16. Traumatized patients who develop the lethal triad of death have a mortality rate up to 50% 17. Both hypothermia and acidosis cause coagulopathy by hampering platelet aggregation, decreasing the activity of clotting factors, and interfering with fibrinolysis 18,19. However, the observed coagulopathy is not only attributed to metabolic decompensation, but also exacerbated by some resuscitation measures, such as excessive crystalloid administration 19,20.
Peripheral vasoconstriction allows a transient hemostasis due to the preservation of circulating volume, which is presumably being lost through the injured tissue 21,22. This effect of trauma has been described in two moments: the first hit, which encompasses the local and systemic reactions caused by the direct energy transferred by the trauma that causes bleeding, hypoxia and tissue damage; the second hit encompasses secondary reactions to injury and metabolic response, including ischemia/reperfusion phenomena, compartment syndrome, infections, among others 23,24.
Initial Approach
The initial assessment of the trauma patient allows to determine the patient's condition, as well as the best timely decisions to be made for subsequent management. According to the Prehospital Trauma Life Support (PHTLS), the initial assessment of the patient should be focused on 25:
Control of major bleeding (X)
Airway (A)
Breathing (B)
Circulation (C)
Disability (D)
Exposure and control of the environment (E)
The first critical step of prehospital care is to be able to identify if the patient has life-threatening bleeding, which can be challenging on initial evaluation. Given this, initiatives have been undertaken to develop tools to detect hemorrhagic shock in a timely fashion, and to take initial steps to slow hemorrhage on the field. The “Stop the Bleed” campaign recommends an estimation of the patient's volume and hemodynamic status through recognition of specific clinical signs 26. These clinical signs should be evaluated together, rather than relying on only one parameter to determine a patient’s hemodynamic status. The proportion of blood lost in relation to the patient's blood volume can, in early stages, induce vital sign changes such as high heart rate, changes in the respiratory pattern or quality of the pulse wave. As blood loss exceeds 15% of blood volume, tachypnea and mucocutaneous pallor are often evident, without changes in blood pressure. With a loss of more than 30%, hypotension, altered mental status and reduced urine output can occur. As such, if a provider waits for hypotension to suspect that a patient is hemodynamically decompensating, it may already be too late for any intervention 27,28. Therefore, it is recommended to have the systolic blood pressure (SBP) as a reference, with the alarm point being a SBP of less than 90 mmHg 25,29,
Initial Management
Phase 0 of DCR, also known as remote DCR, focuses on prehospital interventions for patients with severe trauma. The measures that should be carried out regardless of the resources or geographic location should include bleeding control, permissive hypotension, fluid therapy, temperature control and immediate transfer to a hospital where definitive care can be provided.
Bleeding Control
In a patient with active hemorrhage, bleeding control is the most important intervention. If bleeding persists, metabolic recovery is not possible, which could exacerbate the patient’s metabolic debt and ultimately lead to exsanguination 30.
The “Stop the Bleed” campaign convened by the National Security Council at the White House of the United States in collaboration with the American College of Surgeons in 2015 was a call to action 31. The main objective of this campaign was to empower untrained people to act in the presence of victims of violent traumatic events with life-threatening bleeding by giving them the tools to stop the bleeding at the scene. The first step in bleeding control is identification of an external bleeding site followed by direct control. The first technique employed should be direct pressure. Pressure should be applied to the external bleeding site until it stops, ideally in a sustained manner, on a firm surface, with or without a dressing 32,33. If despite direct pressure on the wound, bleeding does not stop, or if direct pressure is difficult to perform, for example, because a wounds involves a cavity or is at a site of union (such as the armpit, groin or neck), packing of the wound should be performed. This is achieved through the complete insertion of a clean cloth or dressing from the deepest to the most superficial part against resistance, filling the wound. This technique, however, should not replace direct pressure. While ideally a hemostatic dressing would be used as packing material in order to facilitate the formation of clot, any material can be used. Examples of hemostatic dressings include oxidized cellulose, fibrin glue, synthetic adhesives, as well as Zeolite or Chitosan, which are the latest generations of local hemostatic agents 34.
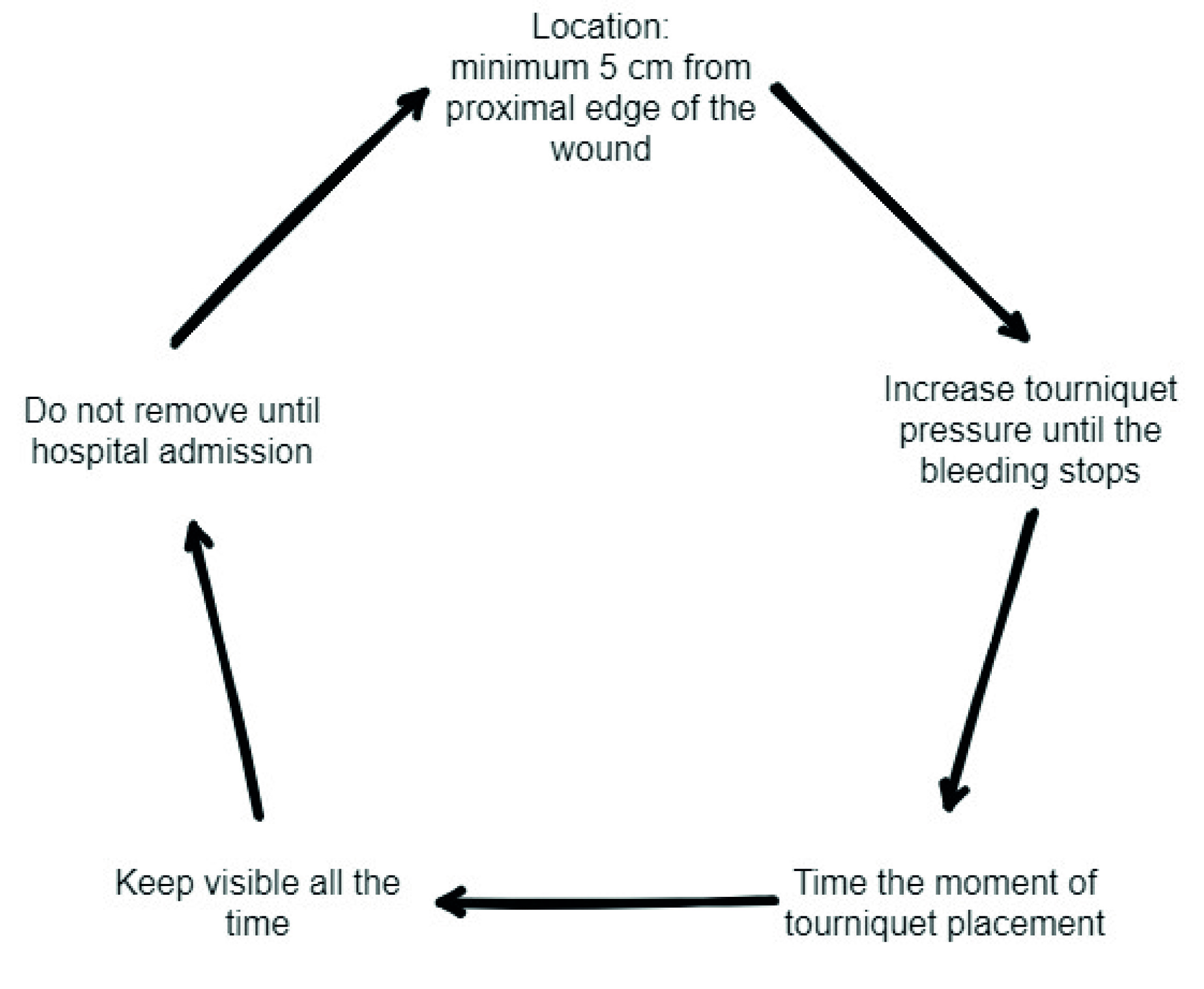
For bleeding in the extremities and for cases that do not involve areas of union, tourniquet should be used as a first line intervention (Figure 1). Although its use has been controversial in the past, current evidence supports its prehospital use in victims of civilian trauma 35,37. In these circumstances, tourniquets have proven to be effective in achieving temporary control of bleeding, with low complication rates. One of the main limitations regarding tourniquet placement is the lack of training. The “Stop the Bleed” course is aimed for the use of combat application tourniquets (CAT), with the caveat that the correct application of these tourniquets is not necessarily transferable to other types of commercially available tourniquets. The main indication for the use of tourniquets is major bleeding from the extremities. When placing a CAT, the following 5 important points should be considered (Figure 2) 38,39:
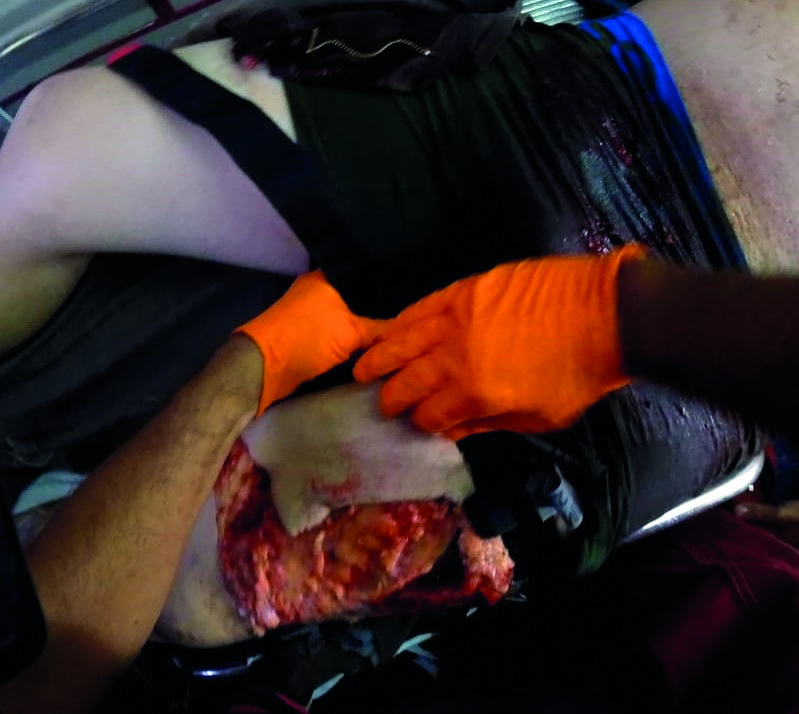
Figure 1 Combat Application Tourniquets being placed on a patient with extremity trauma at the scene.
The Trauma and Emergency Surgery group in Cali (CTE), has been pioneer on the education of both medical and non-medical personnel through the “Stop the Bleed” course throughout Latin America, specifically in Colombia, Costa Rica, and Ecuador. In our first course, with the training of 265 people, we compared if there were any differences if the courses were dictated by surgeons or by medical students. Among personnel with prehospital training prior to the course only 84.2% reported knowing how to apply any bleeding control technique, and only 43.4% stated that they would place a tourniquet in case of bleeding, however, this perception changed after the course, when more than 90% stated that they would likely use a tourniquet. It was found that there was no significant difference between those trained by surgeons vs. those trained by medical students 40,41. In subsequent analyses, we have also explored factors prior to the course that could be associated with correct use of bleeding control strategies on the field. It was identified that the number of previous trainings (having taken prior courses such as Basic Life Support, Advance Cardiovascular Life Support, PHTLS), having previously used the tourniquet, and being non-medical personnel were all factors associated with a greater probability of good use of a tourniquet. The association with nonmedical personnel and good tourniquet use may be due to the fact that tourniquet use has been taught as a harmful technique during medical education, a belief that has been contradicted with recent evidence.
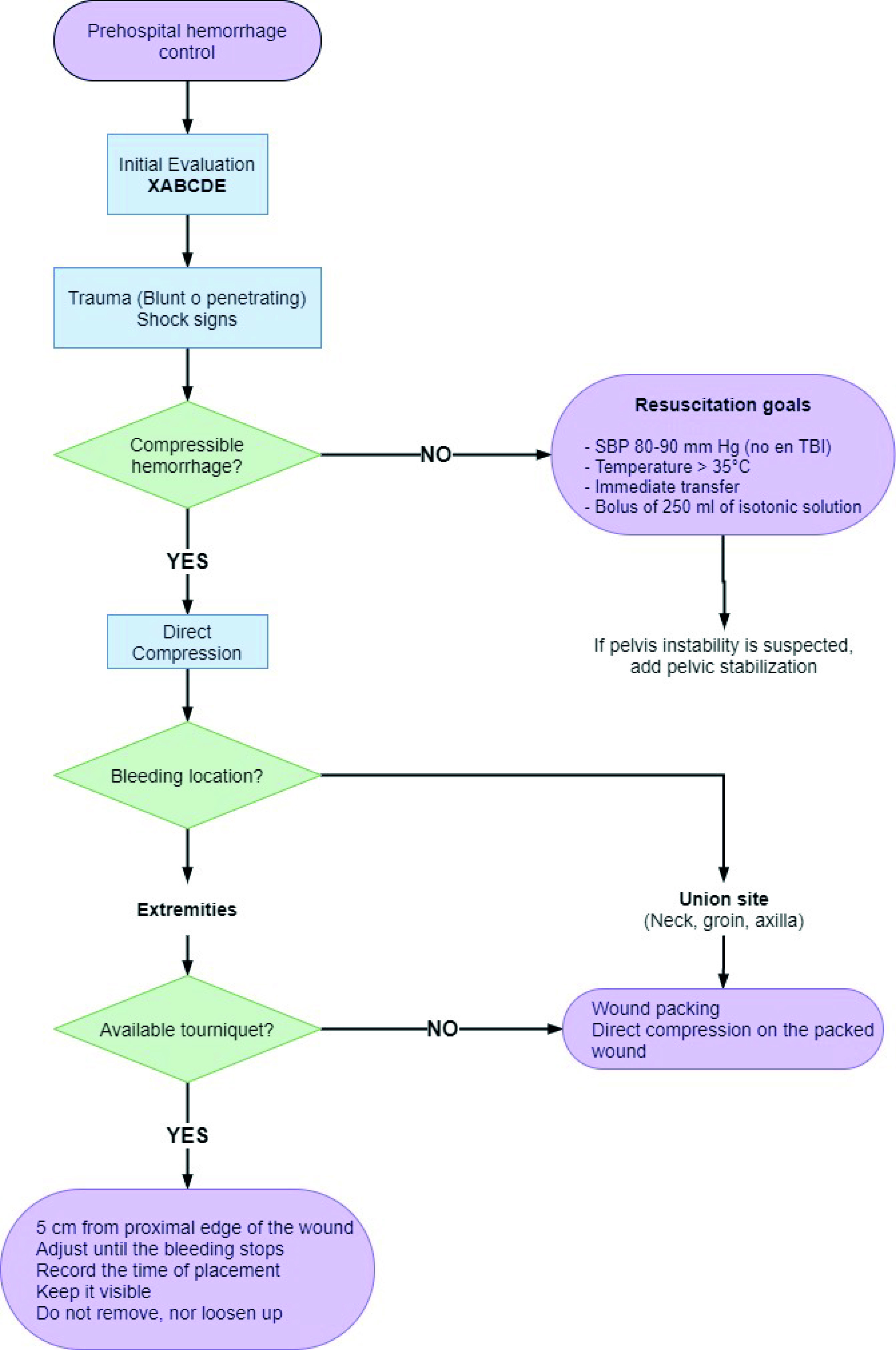
Finally, to facilitate decision-making when managing a patient with bleeding in the prehospital setting, we propose the following management algorithm (Figure 3):
Permissive Hypotension
During World War I, Dr. Cannon observed that administering fluids to trauma patients in hemorrhagic shock could increase bleeding because of clot disruption due to an increase in blood pressure. However, only in the 1990s the concept of permissive hypotension was resume and studied 42. The main objective of this strategy was to decrease the amount of fluids administered to a patient with massive bleeding while achieving surgical hemostasis, thereby maintaining only the minimum blood pressure necessary for the perfusion of vital organs. In 1994, Bickel and Mattox evaluated patients resuscitated with a high-volume crystalloid strategy vs. volume-restricted crystalloid strategy. They found an 8% reduction in mortality in hypotensive patients who were victims of penetrating torso trauma in the resuscitation group with volume restriction compared to the resuscitation group without fluid restriction 43. However, these findings have been difficult to replicate in subsequent studies. In a 2002 study by Dutton et al. 44, it was found that titration of initial fluid therapy to a lower-than-normal SBP value during active bleeding did not affect mortality of patients who were victims of blunt or penetrating trauma. However, it is accepted that infusion should be maintained to keep blood pressure in the range that allows end-organ perfusion.
Fluid Therapy
It has been shown that the type of fluid used for resuscitation has a direct effect on clinical outcomes. There is sufficient evidence to justify the use of crystalloids, as neither colloids nor hypertonic solutions have shown to have a benefit when compared to the infusion of isotonic crystalloids 45-49. The use of blood products such as plasma or even whole blood outside the hospital is not a widely available practice; consequently, it will not be discussed in this section. However, the authors acknowledge that the early control of bleeding and replacement of the lost volume with blood would improve clinical outcome.
Crystalloids are electrolyte-based solutions currently used as the first choice of resuscitation fluid prior to hospital admission. Normal saline (0.9% NaCl) together with Lactate Ringers are the most frequently used crystalloids. One of the main limitations of their use as a resuscitation fluid is that they are very poor expanders of plasma volume, because only about 20% of the infused fluid remain in the intravascular space, and the rest, spread into the interstitial space 50.
To help define situations in which patients with life-threatening bleeding may benefit from crystalloid infusion, the establishment of clear criteria that warrant prehospital resuscitation with intravenous fluid is required. Table 1 summarizes some parameters that can be taken into account when determining which patients could benefit from prehospital volume resuscitation 51-55. It is important to recognize that guiding resuscitation using preset SBP values could lead to the administration of large volumes of crystalloid, which could have serious complications. The main side effects associated with the use of crystalloids include: increase in the bleeding as a consequence of clot disruption, dilutional coagulopathy, electrolyte disturbances, edema, end-organ dysfunction, fluid overload, limb and abdominal compartment syndrome, decreased wound healing, and increased mortality, among other cardiac, respiratory, gastrointestinal and immunological complications 56-60.
Table 1 Clinical criteria to establish the goals of intravenous fluid therapy such as time to hospital arrival, mechanism of trauma, and traumatic brain injury 51-55.
| Parameter | Indication for IV infusion |
|---|---|
| Time to hospital arrival | >10-15min of transport time |
| Mechanism of trauma | Penetrating trauma: SBP <60-70 mmHg |
| Blunt trauma: SBP <80-90 mmHg | |
| Traumatic brain injury (TBI) | Penetrating trauma + TBI: SBP <100-110 mmHg |
To minimize the complications associated with excessive intravenous fluid administration, and to simplify the decision-making process as much as possible in the prehospital setting, our group, in accordance with international guidelines 61,62 recommends administering crystalloids as boluses of 250 cc at a time.
Temperature Control
Hypothermia is an important factor contributing to the worsening of coagulopathy, cardiac function, and metabolic acidosis. There are several potential causes of hypothermia in trauma patients, including: exposure to cold at the site of injury, administration of cold intravenous fluids, and metabolic disorders due to the trauma itself. Hypothermia can be classified into 3 categories: mild (34-36° C), moderate (34-32° C) and severe (<32° C) 8. Although, patients with the worst outcomes are those with temperatures of 32° C or less 63, an increase in mortality can be noted in patients with temperatures lower than 35° C 64. Diverse strategies can be employed to maintain a core body temperature above 35° C in severely injured trauma patients, such as using heated blankets, warming of intravenous fluids to 39° C prior to infusion, and early control of hemorrhage.
Immediate Transfer
Prehospital personnel should never delay the transfer to the hospital, not even for resuscitative interventions on any patient with major bleeding, especially with non-compressible bleeding. Early transfer to the hospital is so important that some studies have found that even when transportation is not performed by emergency medical services, as long as it is immediate, there is an immediate benefit on mortality 65,66. It is crucial that prehospital staff aim to shorten response time, scene time and transfer time to effectively give severe injured patients a timely intervention. The challenge for those delivering prehospital care is to identify circumstances that do justify aggressive out-of-hospital interventions such as tourniquet placement in patients with massive bleeding of an extremity, maintaining a patent airway or starting fluids in patients with crush injuries, among many others.
The Future
The CTE group from Cali, Colombia, is pioneer in innovation and care of trauma patients. As our experience grows with novel techniques such as the use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA), hemostatic resuscitation, and pharmacological supplements, efforts should be made to transfer some of these advances into the prehospital setting. We believe that technology like REBOA is not necessarily the future of trauma, but rather the present. It is important that trauma centers in our region begin to expand upon their own experience with such technologies. With an increasingly advanced world, we believe that stabilization protocols for prehospital personnel in our region, and the implementation of courses to teach basic skills such as arterial line placement in the common femoral artery are just around the corner. The future holds additional challenges as evidence grows around hemostatic resuscitation, supporting the use of whole blood inside and eventually outside the hospital. Finally, we must understand that time is essential in trauma and patients require aggressive and efficient management from the moment that injury occurs. To give our patients the best chance at survival, our trauma systems must be kept up-to-date with current technology, this is why, and especially for cases of long transfer time, we have to consider the implementation of some of these new techniques to improve patient outcomes 67,68.
Conclusion
Prehospital care is the first step to guarantee bleeding control and hemostatic resuscitation of trauma patients. Early interventions without increasing transfer time to a hospital are the keys to increase the success rate of the other strategies used in damage control. Strategies like the “Stop the Bleed Campaign” can provide tools for non-medical personnel to perform early maneuvers that can make a difference and decrease mortality.











 text in
text in