Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.11 no.1 Popayán Jan./June 2013
EVALUACIÓN DE LA CALIDAD DE CARAMELOS DE UCHUVA SIN SACAROSA ADICIONADOS CON CALCIO
QUALITY ASSESSMENT OF CAPE GOOSEBERRY CANDY WITH ADDED CALCIUM WITHOUT SUCROSE
AVALIAÇÃO DA QUALIDADE DE DOCES DE CAMAPÚ SEM SACAROSE ADICIONADA COM CÁLCIO
FRANCIA ELENA VALENCIA G.1, MISAEL CORTÉS R.2, MARÍA ORFILIA ROMÁN M.3
1 Ph.D. Pharmaceutical and Food Science, Universidad de Antioquia, Pharmaceutical Chemistry Faculty, Food Department. GIAS Research Group. Medellin. Colombia.
2 Ph.D. Food Engineering, Universidad Nacional de Colombia, Faculty of Agricultural Sciences, Department of Agricultural and Food Engineering, Medellin-Colombia P.O. Box 568.
3 M. Sc. Chemical, Universidad de Antioquia, Pharmaceutical Chemistry Faculty, Food Department. GIAS Research Group. Medellín. Colombia.
Correspondencia: francia.valencia@gmail.com
Recibido para evaluación: 15/09/2011. Aprobado para publicación: 18/09/2012
RESUMEN
Este trabajo evalúa el efecto de la adición de calcio sobre los atributos de calidad de un caramelo blando a base de fruto de uchuva sin sacarosa, comparándolo con uno similar sin calcio. Se emplea calcio lácteo como ingrediente funcional, el cual se incorpora a granel, con el objetivo de alcanzar un valor diario de referencia mayor del 20% en una porción de 15 g de producto, según la normativa colombiana. El calcio afecta principalmente la acidez del producto (<), el pH (>), la elasticidad (>), la cohesividad (<) y la luminosidad (>). El caramelo adicionado presentó contenidos de 292,5 ± 9 mg Ca/porción, correspondiente a un 20 % del valor diario de referencia, lo cual permite identificar al producto con el descriptor 'Excelente fuente de calcio'. Los caramelos blandos de uchuva sin azúcar adicionados con calcio, representan una alternativa económica para cubrir necesidades de calcio en la dieta de la población.
PALABRAS CLAVE: Physalis peruviana L., Alimentos Funcionales, Confitería, Componentes fisiológicamente activos.
ABSTRACT
The effect of added calcium on the quality attributes of a soft candy made of cape gooseberry fruits without sucrose is assessed by comparing it to a similar candy without calcium. Dairy calcium is used as functional ingredient; it is incorporated in bulk to reach a daily reference value higher than 20% of calcium in a 15 g portion of the product, according to the Colombian regulations. The calcium addition in the candy mainly affects the acidity of the product (<), the pH (>), elasticity (>), cohesiveness (<), and luminosity (>). The candy with added calcium presented contents of 292,5 ± 9 mg Ca/portion, corresponding to 20% of the daily reference value, which makes it possible to identify the product with the following descriptor: 'An excellent source of calcium.' Cape gooseberry soft candies with added calcium and without sugar represent an inexpensive food alternative to fulfill the calcium needs of the populations diet.
KEYWORDS: Physalis peruviana L., Functional food, Confectionery food, Physiologically active components.
RESUMO
Este estudo avalia o efeito da adição de cálcio sob os atributos da qualidade de um doce macio de fruto de camapú sem sacarose, em comparação com um similar sem cálcio. Empleou-se cálcio de origem lácteo como um ingrediente funcional, que é incorporado a granel, a fim de alcançar um valor de referência diária superior ao 20% numa porção de 15 g de produto, de acordo com a lei colombiana. O Cálcio afeta principalmente a acidez do produto (<), pH (>), elasticidade (>), coesão (<) e brilho (>). O conteúdo apresentado no caramelo 292,5 ± 9 mg Ca+2/porção, com 20% do valor de referência diária, que identifica o produto com o descritor 'Excelente fonte de cálcio.'Os doces macios livres de açúcar com adição de cálcio, representam uma alternativa econômica para atender às necessidades de cálcio na dieta da população.
PALAVRAS CHAVE: Physalis peruviana L., Alimentos Funcionais, Confeitaria, Componentes fisiologicamente ativos.
INTRODUCTION
In Colombia, cape gooseberry (Physalis peruviana L.) is a fruit considered to be promissory, and over the past years its export volume and internal market consumption have increased [1]. Such situation is an opportunity for the Colombian growers and industrialists to try to take advantage of the cape gooseberry fruits that do not make the export quality cut and, therefore, to give them added value through their transformation and incorporation with physiologically active components in the development of food products such as confectionery and dehydrated derivatives, beverages, among other products. With this practice, the current food product offering of the national and international markets will be considerably improved, thus, it will contribute to the strengthening of the fruit productive chain and its industry in Colombia [2].
Developing candy becomes a healthy alternative to adding gooseberry pulp and physiologically active components. Soft candies are amorphous gummy structures [3,4] and they can be chewable or not-chewable. Soft candies are the result of cooking a syrup composed by ingredients such as sugar, glucose (or mixes of both), fat, and edible oils; also, they can have added milk and emulsifiers, among other ingredients suitable for human consumption and allowed by the corresponding authorities [5]. Sucrose is the main sweetener used in the production of candy, but its caloric contribution is quite high, which makes such candies unsuitable for diabetic people. By replacing sucrose in this type of food products, the functional qualities are affected; therefore, it must be substituted for compounds such as polydextrose, sorbitol, maltodextrins, artificial sweeteners, among other [6]. Furthermore, it allows the addition of other ingredients fit for human consumption allowed by the competent authority.
Calcium has been widely used as an additive in food to achieve multiple purposes such as the following: interacting with pectins and other components of fruit and vegetable cell walls to obtain products with a higher firmness [7], reducing the browning induced by the polymerase enzyme [8], limiting the sporulation caused by pathogens [9], and improving cheese structure [10]. The interactions that can occur between the added source of calcium and the other ingredients present in the food products (polymers: fibers, pectic acids or proteins) can affect the rheological properties of food and, at the same time, the availability of calcium [11, 12]. Insoluble calcium sources can precipitate in the container and in the equipment surface, causing its deterioration due to the incrustations that appear [13].
Calcium is currently used in the production of functional food [14,15,16,17] because it is a physiologically active component that improves osseous health [18, 19]. The source of calcium must have specific characteristics in terms of technological application methods and food processing; it must have a reasonable price, and it should not interfere in the metabolism of other nutrients, or produce undesirable sensorial effects. The addition of calcium to the food must be controlled by regulations specifying allowed levels in a defined portion [20]. In the case of Colombia, products with added nutrients are regulated by the Ministry of Social Protection, through Resolution 333, which establishes the declaration of nutritional properties with the following descriptors: High, Good source, Free, Low, Very low, Lean and Extra-lean, or synonyms such as: '-rich, Excellent source of, Good source of, Provides, Source of, Contains, or With' [21]. Other criteria reported for the case of calcium as a good source of calcium is the fact that it provides at least 30 mg of absorbable calcium per standard portion, or for every 418 kJ (100 kcal) of food [22].
The bioavailability of the calcium source indicates how much of it is truly absorbed during the digestion process [23]. Calcium sources used in food have different calcium content in mg/g of product, and it can range from 9% to 40% according to the size of the molecules. For example, calcium citrate and calcium carbonate have 50% and 40% of calcium respectively; but due to the fact that the size of the citrate molecule is considerably big, only 10,5% of the calcium is available, while 25% of calcium is available in the case of calcium carbonate [26]. The following are some of the calcium sources used to enrich milk, beverages, and other food products: calcium carbonate, calcium chloride, calcium phosphate, tricalcium phosphate, calcium citrate malate, calcium lactate, calcium gluconate, calcium lactate gluconate, and dairy calcium [14,25].
The objective of this research project consists in assessing the quality attributes of a cape gooseberry soft candy with added calcium and without sucrose by comparing it to a similar candy without calcium.
METHOD
Raw materials. For this research, fresh Colombia ecotype cape gooseberry fruits were used, which were grown in La Union, Antioquia. The selected cape gooseberry fruits were in the range of 3 - 4 in the color scale according to the commercial ripeness as it is established by the Colombian Technical Regulation NTC 4580 [26]. Pulp extraction was performed according to the method established by Echeverri [27]. Dairy calcium (with a purity percentage of 26,5) was bought in the local market, and used as the source of calcium.
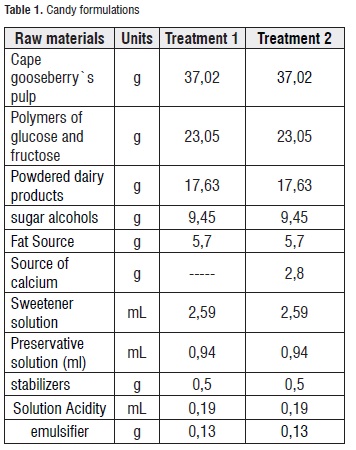
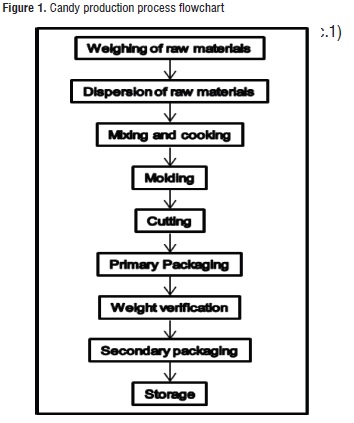
Production Process of the Candy. Two formulations (each batch of 47 Kg) of soft candy were produced, both without sucrose but one without added calcium and the other with added calcium (treatment 1 and 2, respectively). Table 1 shows the studied formulations, and Figure 1 presents the candy production process flowchart.
A 200 L stainless steel cooking pot, a scratching agitator (60 RPM), and a direct transfer gas burner were used. The thermal treatment was performed until the ingredient mix reached 92°C; then, the mix was molded in plastic trays covered with transparent polyethylene bags, and stored in a room where they were left to stand at 28 ± 3°C for 24 hours. Later, the manual cutting process of 5,1 – 5,5 g portions was performed. The product was packaged in an automatic pillow-shaped KD-260 packaging machine, using plates metalized with three layers: biaxially oriented polypropylene (BOPP), aluminum foil, and low-density polyethylene (PEBD), for a total grammage of 55 g/m2. The secondary package consists of BOPP and polyethylene bags with a gauge of 60 µ, and a grammage of 71 g/m2. Eight units were packaged per bag, and then the bag was thermally sealed with a pedal sealing machine. The sealed bags were stored at room temperature (≈ 25°C) until the analyses were performed.
Physicochemical Analyses. The moisture content (% p/p) was determined by heating the product with a stove at 105°C for 5 hours until the constant weight was reached. The method used was the 966.02 gravimetric of the AOAC International [28]; soluble solids (°Brix) measuring the refraction index of the samples diluted at 1% with the HANNA HI 96801 refractometer set in a scale of 0 - 85 °Brix at 20°C. In order to obtain the total value of the samples °Brix, equation 1 was used.

Where L = refractometer reading of the solutions soluble solids, and C = solution concentration (5%).
pH was measured in a HANNA HI 9025 pH-meter previously calibrated with buffer solutions 4 and 7 at 25°C. Total acidity by potentiometric titration at pH 8,1 [29], expressing the results as g of citric acid/100 mL. Calcium was determined by means of the AOAC 985.35 Ed 16 atomic absorption method ?38?, and the water activity (aw) with an AquaLab Decagon CX-3 AOAC 978.19Bc drew-point hygrometer [30].
Physical Properties. The texture profile analysis (TPA) was performed in the TA-XT2i texture analyzer (Stable Micro Systems), equipped with a 50 kg charge cell, and a 100 mm-diameter compression plate. Operation speeds for pre-assay, assay, and post-assay were 9, 10, 9 mm/s respectively; the candy relative deformation was set to 80%, and the time between compressions was 0,8 s. The contact area for the candies was a 30 mm2 square. TPA parameters (hardness, tackiness, elasticity, cohesiveness, compression, gumminess, chewiness, and spreadability) were determined from the strength vs. time graphic [31], which was provided by the Texture Expert Exceed software.
Color measurement was performed using the X-Rite SP-64 portable sphere spectrophotometer, D65 illuminator, and observator 2°, with a built-in specular and a 4 mm observation window. Color coordinates CIE-L*a*b* were determined from the reflection spectra.
Microstructural Analysis and Energy Dispersive Spectroscopy. The scanning electron microscopy technique (SEM) was used, performing it with a JEOL JSM 5950 LV microscope at a vacuum condition of 25 Pa, and 15 kv. Simultaneously, the Energy Dispersive Spectroscopy (EDS) technique was performed; this technique allows obtaining the compositional microanalysis and calcium relative quantities (among others) of the calcic raw material and treatments. This technique poses the advantage of allowing to analyze specific areas in the surface, and it comes pre-installed in the JEOL equipment [32].
Statistical Analysis. A factorial design was made with one factor (formulation), which had two levels: with and without calcium (treatment 1 and 2). Physicochemical and physical variables (rheology and color) were determined in two replicas with 3 and 8 repetitions/lot, respectively. Each replica was the result of a pool of 10 candy samples of 5 g/sample. Results were assessed according to the ANOVA, using the LSD (Least Significant Differences) procedure as a multiple comparison method, with a 95% confidence level (α=0,05) and an 85% power level. The analysis of variance was performed with the 5.1 Statgraphics Plus Statistical Software.
RESULTS
Physicochemical Analyses. Table 2 shows mean values, standard deviation and analysis of variance of the candies physicochemical analyses for the candies without and with added calcium. Moisture, °Brix, and aw did not show significant differences (p>0,05) regarding the treatment factor due to the high variability coefficients that the samples presented; while in the case of acidity and pH, there were significant differences (p>0,05).
Acidity in treatment 1 was higher than in treatment 2, which is consistent with the lowest pH. These differences can be attributed to the hydrolysis and dissociation of the dairy calcium complex, where the calcium citrate predominates. Calcium citrate generates a strong base of calcium hydroxide, which has the shock-absorbing capacity of the acids supplied by the cape gooseberry pulp, reducing proton concentration [12]. Some authors have reported the shock-absorbing effect of calcium over the acidity in the production process of different food products [12, 17].
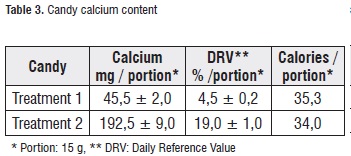
Table 3 shows the mean values and standard deviation for the calcium concentration in the treatments. Treatment-2 candies presented a calcium content equivalent to the 400% of treatment 1, which corresponds to a DRV% of approximately 20% in a 15 g portion. Therefore, in the nutritional properties of this product, the following descriptors can be declared: 'excellent source of calcium' or 'good source of calcium' [21].
Texture Profile. Table 4 shows mean values, standard deviation and analysis of variance of the candies TPA for the applied treatments. Hardness, gumminess and chewiness, did not present significant differences (p>0,05) regarding the treatment factor, while springeness and cohesiveness did present significant differences (p<0,05).
Treatment-2 candies presented higher elasticity, which can be attributed to the interactions of calcium as divalent cation with the other components of the candy (protein, fiber, carbohydrates, among others). These interactions have been exposed by several authors who have researched the linking capacity of casein with calcium through the residues of phosphoserine, with the formation of bridges that tend to cause growth and aggregation of the submicelles ?33?. Such situation is affected by factors like pH, temperature, ionic strength and concentration of calcium ions [34]. The previously described facts could cause that treatment-2 candies have a higher recovery rate than treatment-1 candies after eliminating the distorting strength. The elastic characteristic of the treatments could also be conferred to the presence of sorbitol as plasticizing agent in the system, which is a fact that several authors have noted [35, 36].
Cohesiveness, defined as the texture attribute that refers to the food product deformation rate before it breaks [37], was higher in treatment-1 candies. Such adherence is probably provided to the system by the addition of sorbitol [6] because it is in a medium with a low water availability. In treatment 2, cohesiveness was affected possibly by the greater ionic strength that the system experiences due to the presence of the calcium salt ions. This characteristic causes that treatment-2 candies tend to fall apart easier than treatment-1 candies [38].
Color Analysis. Table 5 shows mean values, standard deviation and analysis of variance of the measurements of the CIE-L*a*b* color coordinates for the applied treatments to the candies. a* and b* color coordinates do not show significant differences (p>0,05) regarding the treatment factor, while L* color coordinate does show significant differences (p<0,05).
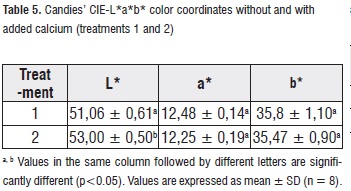
Treatment-2 candies presented lower values in the L* parameter than treatment-1 candies (samples with lighter color and creamy tone); this is attributed to the higher calcium content (white color) in the formulation [13]. Such situation contrasts with the photographs shown in Figure 2.
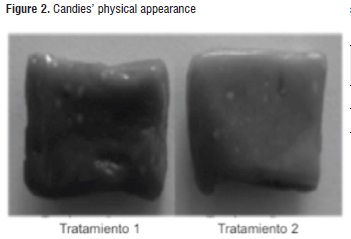
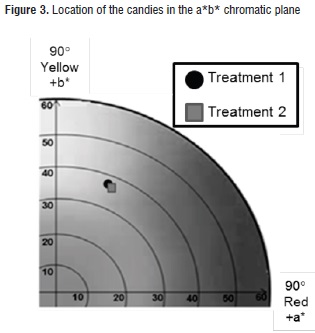
In general, both candies showed a very similar creamy color tonality, which was contributed by the cape gooseberry pulp. According to the chromatic plane a*b* (Figure 3), such color is located near the limits of the yellow, grey and carrot zones, with tone values (hab*) for treatments 1 and 2 of 71,3 ± 1,8 and 69,9 ± 2,5; and chroma or saturation values (Cab*) of 38,4 ± 2,8 and 36,8 ± 3,2, respectively. Similar behaviors have been observed in powdered cape gooseberry obtained through atomization drying [48].
Micro-Structural Assessment
Figure 4 shows micrographs of the used calcium source at 500x and 1000x (Figures 7a and 7b), treatment-1 candies (Figures 7c and 7d), and treatment-2 candies (Figures 7e and 7f). The calcium source showed densely and irregularly packed agglomerates, formed by calcium and other components such as dairy protein, carbohydrates, fat, and phosphorus among others, with sizes ranging from 10 to 40µ. A microstructure with a homogeneous folding shaped surface was observed in the candies of both treatments 1 and 2, but the shape was slightly more regular in those of treatment 2. This microstructure can be due to the plasticizing effect of the sorbitol in the system [45], and a good dispersion of the components in the polymer matrix, in which fat particles suspended in the network stood out. These interactions could contribute to increasing the candy stability and protecting the fat particles against oxidation [43].
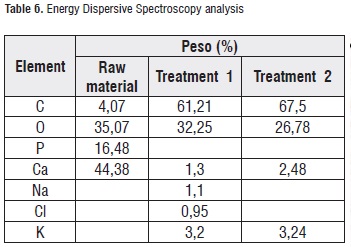
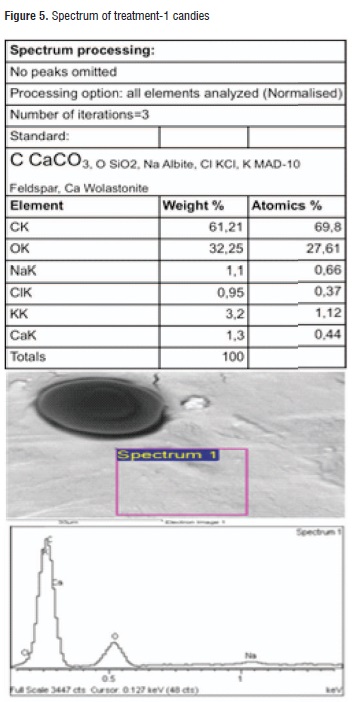
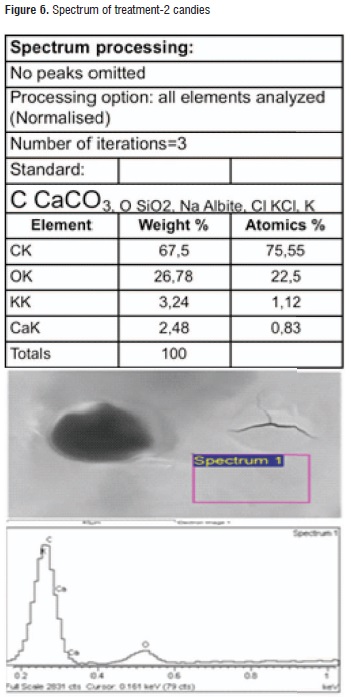
Table 6 shows the results of the EDS analysis obtained for the calcic raw material surface and both applied treatments. It can be observed that common elements that are present in a higher proportion are oxygen, carbon, and calcium representing almost 50% of the material content, as it was expected. Regarding the treatments, the highest calcium content was found in treatment-2 candies. Figures 5 and 6 illustrate the spectra for both treatments.
CONCLUSIONS
Calcium, as a functional ingredient in the production process of cape gooseberry candies, positively affects some product properties, mainly the acidity, pH, elasticity, cohesiveness, and color (L * parameter with the highest significance).
The production of cape gooseberry soft candies with added calcium and without sucrose represent a viable and healthy technological food alternative, which could contribute to the strengthening of the fruit productive chain and the confectionery industry sector, and at the same time, it could help to prevent health issues among its consumers.
The microstructure of the soft candies with added calcium and without sucrose evidenced the food matrix of plasticizing character, in which its components are dispersed and protected in the polymer network.
ACKNOWLEDGEMENTS
The authors of this paper would like to thank the Ministry of Agriculture and Rural Development; food product company KONFYT; Universidad de Antioquia; and the Universidad Nacional de Colombia, Medellín Campus for their support in the execution of this project.
REFERENCES
[1] LETERME, P., BULDGEN, A., ESTRADA, F. and LONDOÑO, A.M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chemistry, 95 (4), 2005, p. 644-652. [ Links ]
[2] COLOMBIA. AGRONET. Cultivo y comercialización de uchuva. Colombia [online]. Available: http://www.agronet.gov.co/www/docs_si2/Cultivo%20de%20uchuva.pdf [Citado 5 de junio de 2011] [ Links ].
[3] GUTIERREZ, E.E., MARTINEZ, A.O., ROMAN, M.O. CADAVID, M. FLOREZ, O.A. y MEDINA, G.B. Elaboración de confites con Fibra de Maracuyá. Memorias V Congreso Internacional Alimentario. Medellín (Colombia): 2007. [ Links ]
[4] SPACKMAN, C. and SCHMIDT, S. Characterising the physical state and textural stability of sugar gum pastes. Food Chemistry, 119 (2), 2010, p. 490–499. [ Links ]
[5] INSTITUTO COLOMBIANO DE NORMAS TÉCNICAS (ICONTEC). NTC 3207: Primera actualización. Productos Alimenticios, azúcar y melaza y productos de confitería, confites blandos. Bogotá (Colombia): 1996, 8 p. [ Links ]
[6] VALENCIA, F.E., MILLÁN, L. y RAMIREZ, N. Evaluación de los efectos en las propiedades fisicoquímicas, sensoriales y texturales de polidextrosa, fructosa y sorbitol como sustitutos de azúcar en la elaboración de arequipe. Revista Lasallista de Investigación, 5 (2), 2008, p. 20 – 27. [ Links ]
[7] FENNEMA, O. Química de los alimentos. Madrid (España): Acribia, 2000. [ Links ]
[8] BARRERA, C., BETORET, N. and FITO, P. Ca2þ and Fe2þ influence on the osmotic dehydration kinetics of apple slices var. Granny Smith. Journal of Food Engineering, 92 (4), 2004, p. 9–14. [ Links ]
[9] CARLIN, F. Origin of bacterial spores contaminating foods. Food Microbiology, 28 (1), 2011, p. 177-182. [ Links ]
[10] WALSTRA, P. Ciencia de la leche y Tecnología de productos lácteos. Madrid (España): Acribia, 2001. [ Links ]
[11] SCILINGO, A.A. and AÑÓN, M.C. Characterization of soybean protein isolates: the effect of calcium presence. Journal of the American Oil Chemists' Society, 81 (1), 2004, p. 63 – 69. [ Links ]
[12] CÁCERES, E., GARCÍA, M.L. and SELGAS, M.D. Design of a new cooked meat sausage enriched with calcium. Meat Science, 73 (2), 2006, p. 368–377. [ Links ]
[13] VALENCIA, F.E. Asuntos varios: Fragmento de compilación intelectual de docentes de la Corporación Universitaria Lasallista: Desarrollo de Alimentos Light. 1 ed. Medellín (Colombia): Marín Vieco Ltda, 2003, 150 p. [ Links ]
[14] TOBELMANN, R. Implementing Calcium Fortification: An Industry Case Study. Journal of Food Composition and Analysis, 14 (3), 2001, p. 241-244. [ Links ]
[15] CERKLEWSKI, F. Calcium fortification of food can add unneeded dietary phosphorus. Journal of Food Composition and Analysis,18 (6), 2005, p. 595–598. [ Links ]
[16] CORTÉS, R.M., GARCÍA, S.A. y SUÁREZ, M.H. Fortificación de hongos comestibles (Pleurotus ostreatus) con calcio, selenio y vitamina C. Revista Vitae, 14 (1), 2007, p. 16 -24. [ Links ]
[17] SINGH, G. and MUTHUKUMARAPPAN, K. Influence of calcium fortification on sensory, physical and rheological characteristics of fruit yogurt. LWT- Food Science and Technology, 41 (7), 2008, p. 1145–1152. [ Links ]
[18] VALENCIA, F.E., CARDONA, D.P. y ROMÁN, M.O. El calcio en el desarrollo de alimentos funcionales. Revista Lasallista de Investigación, 8(1), 2011, p. 123–134. [ Links ]
[19] GRADOS, F., BRAZIER, M., KAMEL, S., DUVER, S., HEURTEBIZE, N., MAAMER, M., MATHIEU, M., GARABÉDIAN, M., SEBERT, J.L. and FARDELLONE, P. Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint Bone Spine, 70 (3), 2003, p. 203–208. [ Links ]
[20] KESSENICH, C. Alternative Choices For Calcium Supplementation. The Journal for Nurse Practitioners, 4(1), 2008, p.36 – 39. [ Links ]
[21] COLOMBIA. MINISTERIO DE PROTECCIÓN SOCIAL. Resolución 333 de 2011: Requisitos de rotulado o etiquetado nutricional. Bogotá (Colombia): 2011. [ Links ]
[22] TITCHENAL, A. and DOBBS, J. A system to assess the quality of food sources of calcium. Journal of Food Composition and Analysis, 20(8), 2007, p. 717–724. [ Links ]
[23] PATWARDHAN, U., PAHUJA, D. and SAMUEL, A.M. Calcium bioavailability: an in vivo assessment. Nutrition Research, 21 (4), 2001, p. 667–675. [ Links ]
[24] AUGSPURGER, N. and BAKER, D. Phytase improves dietary calcium utilization in chicks, and oyster shell, carbonate, citrate, and citrate-malate forms of calcium are equally bioavailable. Nutrition Research, 24 (4), 2004, p. 293–301. [ Links ]
[25] LAWRENCE, F. Multiple sources of dietary calciumsome aspects of its essentiality. Regulatory Toxicology and Pharmacology, 39 (2), 2004, p. 67–80. [ Links ]
[26] INSTITUTO COLOMBIANO DE NORMAS TÉCNICAS (ICONTEC). NTC 4580: Frutas frescas. Uchuva. Especificaciones. Bogotá (Colombia): 1999. [ Links ]
[27] ECHEVERRI, E. Diseño y desarrollo a escala piloto de caramelos blandos a base de fruto de uchuva (Physalis peruviana L.) adicionados de calcio, vitamina D y fibra dietaria como un alimento funcional [Tesis M.Sc. en Ciencias Farmacéuticas]. Medellín (Colombia): 2012, 108 p. [ Links ]
[28] A.O.A.C. International Official Methods of Analysis of AOAC International. 17 ed. Maryland (United States of America): 2003. [ Links ]
[29] KIRK, R.S., SAWYER, R. y EGAN, H. Composición y Análisis de los Alimentos de Pearson. 2 ed. México: C.E.C.S.A., 2000. [ Links ]
[30] A.O.A.C. International Official Methods of Analysis of AOAC international. 16 ed. Arlington (United States of America): 1991. [ Links ]
[31] ALVARADO, J.D. Métodos para medir propiedades físicas en industrias de alimentos. Madrid (España): Acribia, 2000. [ Links ]
[32] YÉVENEZ, J., SILVA, E. y ESCOBAR, I. Formación y control del crecimiento de películas de óxido de aluminio crecidas sobre aluminio 6061 [Online]. Available: http://cabierta.uchile.cl/revista/28/articulos/pdf/paper7.pdf [citado 7 de junio de 2011] [ Links ].
[33] DICKINSON, E. and GOLDING, M. Influence of calcium ions on creaming and rheology of emulsions containing sodium caseinate. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 144 (1-3), 1998, p. 167-177. [ Links ]
[34] MEKMENE, O. and GAUCHERO, F. Determination of calcium-binding constants of caseins, phosphoserine, citrate and pyrophosphate: a modelling approach using free calcium measurement. Food Chemistry, 127 (2), 2011, p. 676-682. [ Links ]
[35] FABRA, M., TALENS, T. and CHIRALT, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid–beeswax mixtures. Journal of Food Engineering; 85 (3), 2008, p. 393–400. [ Links ]
[36] ARRIETA, M.P., PELTZER, M.A., GARRIGOS, M.C. y JIMENEZ, A. Caracterización de las biopelículas Biopelículas activas obtenidas a partir de proteínas lácteas: Envases alimentarios sostenibles Medio Ambiente, 31 (121), 2011, p. 47 -56. [ Links ]
[37] OSORIO, J., CIRO, F., VELÁSQUEZ, Y.H. y MEJÍA, L. Caracterización reológica y textural del queso Edam [Online]. Available: http://redalyc.uaemex.mx/redalyc/src/inicio/ArtPdfRed.jsp?iCve=49614705 ISSN 0012-7353, 2005. [citado 7 de julio de 2011] [ Links ].
[38] HERNANDEZ, G. Desarrollo de un producto de uchuva (Physalis Peruviana L.) en polvo secado por atomización adicionado con vitamina C, ácido fólico, hierro y fibra soluble [Tesis M.Sc. En Ciencia y Tecnología de Alimentos]. Medellín (Colombia): 2011, 78 p. [ Links ]













