Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.16 no.2 Bogotá July/Dec. 2012
Surfactant modified clays’ consistency limits and contact angles
Suat Akbulut, Z. Nese Kurt and Seracettin Arasan1
Ataturk University, Civil Engineering Department, 25240 Erzurum, Turkey.
1 Corresponding author. Phone: + 90 4422314618; fax: + 90 4422360957. E-mail: arasan@atauni.edu.tr (S. Arasan).
Record
Manuscript received: 14/10/2011 Accepted for publications: 06/05/2012
ABSTRACT
This study was aimed at preparing a surfactant modified clay (SMC) and researching the effect of surfactants on clays’ contact angles and consistency limits; clay was thus modified by surfactants for modifying their engineering properties. Seven surfactants (trimethylglycine, hydroxyethylcellulose, octyl phenol ethoxylate, linear alkylbenzene sulfonic acid, sodium lauryl ether sulfate, cetyl trimethyl ammonium chloride and quaternised ethoxylated fatty amine) were used as surfactants in this study. The experimental results indicated that SMC consistency limits (liquid and plastic limits) changed significantly compared to those of natural clay. Plasticity index and liquid limit (PI-LL) values representing soil class approached the A-line when zwitterion, nonionic, and anionic surfactant percentage increased. However, cationic SMC became transformed from CH (high plasticity clay) to MH (high plasticity silt) class soils, according to the unified soil classification system (USCS). Clay modified with cationic and anionic surfactants gave higher and lower contact angles than natural clay, respectively.
Keywords: Consistency limit, contact angle, surfactant, clay.
RESUMEN
Este estudio tiene como objetivo la preparación de un surfactante de arcilla modificada (SMC) y la investigación de sus efectos en los ángulos de contacto y los límites de consistencia de las arcillas, es decir, sus propiedades de ingeniería. Siete surfactantes (trimetilglicina, hidroxietilcelulosa, octil fenol etoxilato, ácido lineal alquilbenceno sulfónico, sulfato sódicol auril éter, cloruro de cetil trimetil amonio cuaternario y amina grasa etoxilada) fueron utilizados como en este estudio. Los resultados experimentales indicaron que los límites de consistencia de SMC (límites líquido y plástico) cambiaron significativamente en comparación con los de arcilla natural. El índice de plasticidad y límite líquido (PI-LL) que representan el tipo de suelo, se acercaron a la línea A cuando aumentó el porcentaje de zwitterion, nonionic y el surfactante aniónico. Sin embargo, catiónicos SMC se transformaron a partir de CH (arcilla de alta plasticidad) a los suelos de la clase MH (limo alta plasticidad), según el sistema de clasificación de suelos unificado (USCS). La arcilla modificada con surfactantes catiónicos y aniónicos dio mayores y menores ángulos de contacto que la arcilla natural, respectivamente.
Palabras claves: Límite de cosistencia, ángulo de contacto, surfactante, arcilla. .
Introduction
Due to their low permeability, clays are the main material used as a liner in solid waste disposal landfills. They become exposed there to various chemical, biological and physical events and the clay is affected by the resulting leachate (Yılmaz et al., ., 2008); researchers are thus interested in surfactants and polymers for modifying clays’ engineering properties. Surfactants are surface-active agents which alter fluid interface properties. Surfactant modified clay (SMC) or surfactant clay complexes have been considered appropriate landfill liners (Lo, 2001; Ashmawy et al., ., 2002; Gates et al., ., 2004; Matott et al., ., 2006) and also potential sorbents for wastewater and contaminated soils (Zhu and Zhang, 1997; Mulligan et al., ., 1999a, b; Mulligan et al., ., 2001; Al-Asheh et al., ., 2003; Li et al., ., 2003; Wibulswas, 2004; Ghiaci et al., ., 2004; Yang et al., ., 2005).
Consistency limits (Atterberg limits) have been repeatedly shown to be useful indicators of clay behaviour (Jefferson and Rogers, 1998; Do- linar et al., ., 2007). Reconstituted clay sample liquid and plastic limits can be correlated with various engineering properties, such as specific surface area, cation exchange capacity, permeability, shrinking and swelling behaviour, shear strength and soil compressibility (Sharma and Lewis, 1994; Abdullah et al., ., 1999; Yukselen and Kaya, 2006; Dolinar et al., ., 2007). Consequently, soil consistency limits are determined, then some other geotechnical properties whose determination may take a long time can be easily estimated with acceptable accuracy. Evaluating consistency limits provides some very basic mechanical data about a particular soil and a first insight into a particular clay’s chemical reactivity. The liquid limit and plasticity index are mainly influenced by clay minerals’ ability to interact with liquids (Schmitz et al., ., 2004) and hydraulic conductivity tends to decrease when the liquid limit and plasticity index become increased (Alawaji, 1999; Met et al., ., 2005; Arasan and Yetimoglu, 2008).
Contact angle measurements are often used to indicate clay wettability and interfacial tension (Rogers et al., ., 2005) and provide an insight into surfactant behaviour. The pertinent literature contains only a limited number of studies on SMC contact angles (Jouany and Chassin, 1987; Janczuk et al., ., 1989; Lopez-Duran et al., ., 2003; Cipriano et al., ., 2005; Dharaiya and Jana, 2005). Rogers et al., ., (2005) examined the contact angles of some compatibilisers for polymer-silicate layer nanocomposites. Dharaiya and Jana (2005) and Cipriano et al., ., 2005) have also investigated SMC contact angles. Cipriano et al., ., (2005) observed that imidazolium bF4 surfactant becomes adsorbed onto a mica surface, resulting in a hydrophobic surface. Similarly, Jouany and Chassin (1987) indicated that a cationic surfactant will become adsorbed with its positively-charged head group next to the negatively-charged clay surface, thereby forcing the hydrophobic surfactant tail to adsorb and become exposed to the solution. Thus, while native montmorillonite surface is hydrophilic, adsorption of a small amount of surfactant on the surface can render it hydrophobic.
Many studies can be found dealing with surfactant (anionic, cationic and nonionic) modified clays (Fu and Qutubuddin, 2000; Gungor et al., ., 2001; He et al.,., 2005; Isci et al., ., 2008; Guegan et al., ., 2009; Gurses et al., ., 2009). Most have been focused on investigating SMCs’ electrokinetic properties, such as zeta potential, cation exchange capacity, electrical conductivity, etc; however no comprehensive study has been found comparing SMC contact angles and consistency limits. This paper was, thus aimed at investigating the effect of seven surfactants (zwitterion, nonionic, anionic, and cationic surfactants) on smectite clay’s consistency limits and contact angles. The surfactants used in suspensions were 5% (250 ppm), 10% (500 ppm), and 15% (750 ppm) dry natural clay by weight.
Materials and Methods
Clays
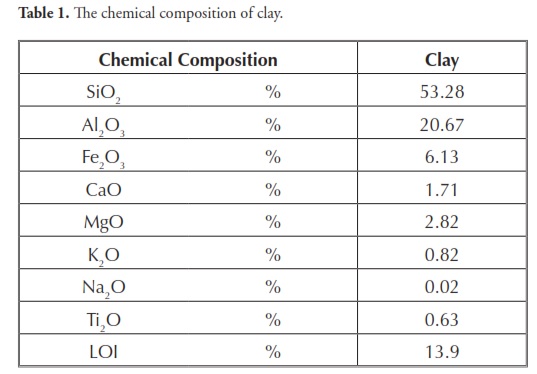
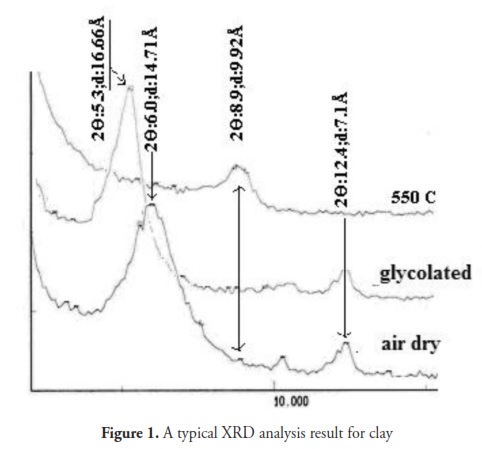
The clayey soil sample used came from a clay-pit in the Oltu-Narman deposits in Erzurum, Turkey, classed as high plasticity clay (CH) according to the unified soil classification system (USCS). These deposits are concentrated in two different stratigraphic horizons, namely late Oligocene and early Miocene sequences. Clay-rich fine-grained sedimentary units were deposited in shallow marine and lagoon-type mixed environments. Their clay minerals originated from the alteration of Eocene calc-alkaline islandarc volcanics, mainly from pyroclastics (trachite and andesite flow) which form the basement for the Oltu depression (Kalkan and bayraktutan, 2008). X-ray diffraction (XRD) and X-ray fluorescence (XRF) methods (Table 1) have been used to identify the major minerals and chemical compounds present in these clays. The soil’s chemical compositions and XRD patterns are given in Table 1 and Fig1, respectively.
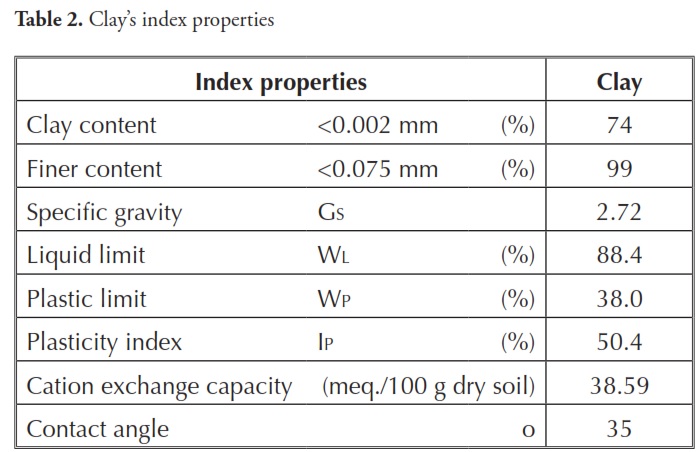
Table 2 gives some of the clay’s index properties. Smectite (56%) appeared to be the predominant clay mineral according to X-ray diffraction (Fig 1), whereas kaolinite (34%) and illite (3%) appeared in lower proportions. Silt and sand-size particles were composed of quartz (4%) and feldspar (3%).Surfactants and modified clay preparation
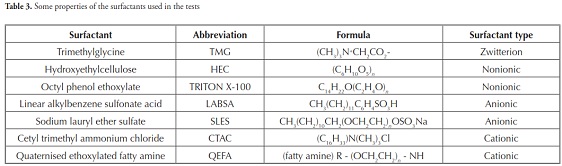
Seven surfactants were used in this research; Table 3 gives the surfactants’ abbreviation, formula and type. SMCs were prepared following the procedure described by Xi et al., ., (2007) and Liu et al., ., (2008). briefly, 40 g of clay was first dispersed in 8 l of deionised water, then stirred with a magnetic stirrer at about 1,000 rpm for about 2 h. Previously-prepared surfactant solution was slowly added to the clay suspension at 30 C. All modified products were dried at room temperature. The surfactants were used at 5% (250 ppm), 10% (500 ppm), and 15% (750 ppm) of clay by weight.
Consistency limits
Consistency limit tests (i.e. liquid limit and plastic limit) were carried out following the procedure outlined in bS 1377, Part 2, 1990. The cone penetrometer (fall cone) method was used to determine liquid limit. Specimens were prepared for the liquid limit tests by mixing an air-dried SMC mass (passing through a 425-μm sieve). Plastic limit tests were performed on material prepared for the liquid limit test; both liquid and plastic limit tests were conducted at room temperature.
Contact angle measurements
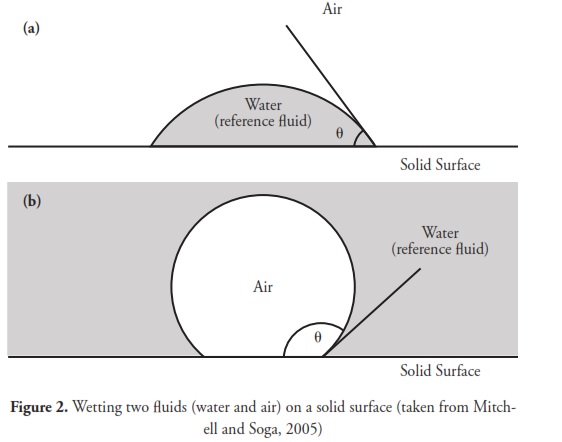
A contact angle is defined as being the angle made by the liquid/solid interface intersection with the liquid/air interface; alternately, it can be described as being the angle between a solid sample’s surface and the tangent of a droplet’s ovate shape at the edge of the droplet. A high contact angle indicates low solid surface energy or chemical affinity; this is also referred to as a low degree of wetting. A low contact angle indicates high solid surface energy or chemical affinity and a high, or sometimes complete, degree of wetting (Anonymous, 2009). Fig. 2 illustrates a drop of the reference liquid (water for Fig. 2a and air for Fig. 2b) resting on a solid surface in the presence of another fluid (air for Fig. 2a and water for Fig. 2b). The interface between the two fluids meets the solid surface at contact angle 0(Mitchell and Soga, 2005).
The simplest way of measuring a contact angle is with a goniometer which allows the user to measure the contact angle visually. The droplet is deposited by a syringe pointing down vertically onto the sample surface and a high-resolution camera captures the image which can then be analysed either by eye (with a protractor) or using image analysis software. The contact angles were measured with a goniometer (CAM 101, KSV Instruments, Finland) in this study, using clay and modified clay pellets.
Results and Discussion
Consistency limits
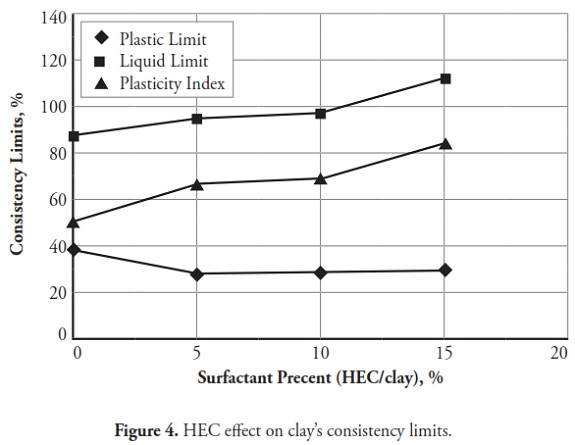
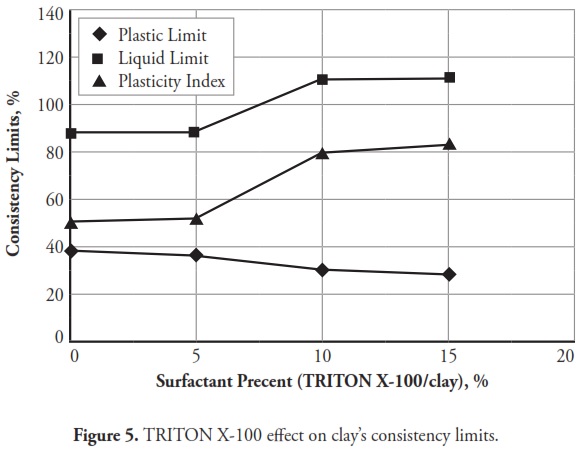
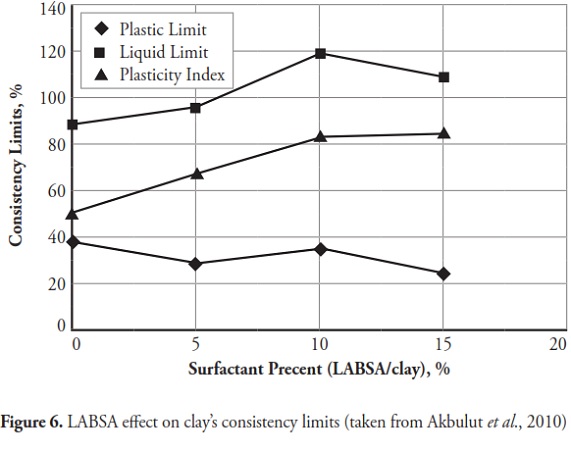
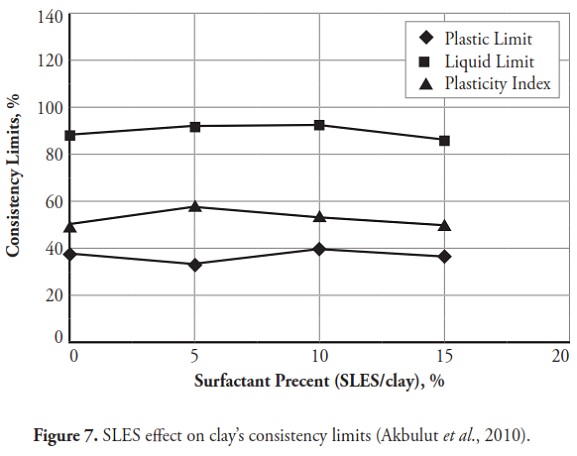
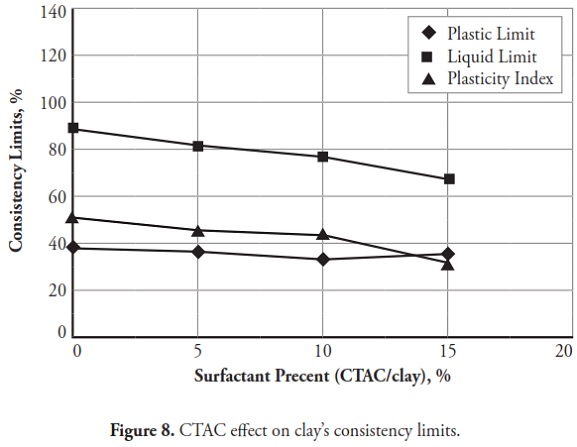
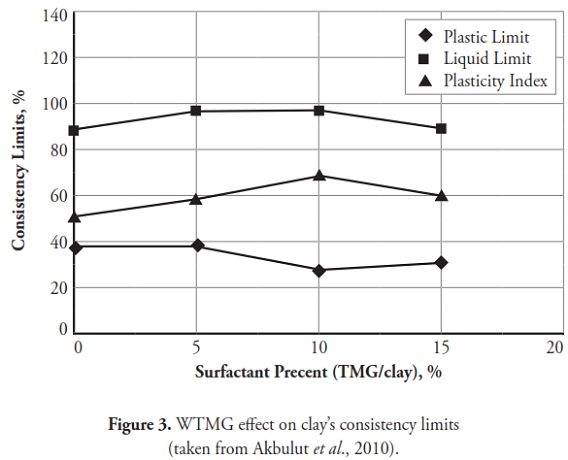
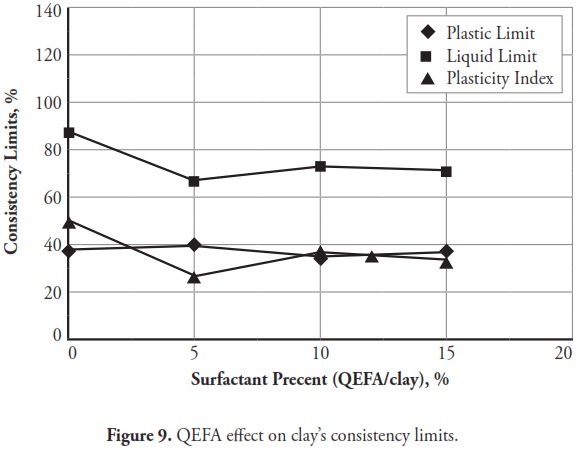
Figures 3 ,4,5, 6,7, 8,9, give the variation of modified clays’ consistency limits by TMG, HEC, TRITON X-100 LAbSA, SLES, CTAC, and QEFA, respectively. For clays modified with zwitterion, nonionic and anionic surfactants (TMG, HEC, TRITON X-100, LAbSA and SLES), it was seen that the liquid limit and plasticity index increased when surfactant percentage was increased. The plastic limit also decreased when increasing the percentage of surfactant. On the other hand, the liquid limit and plasticity index for clays modified with CTAC and QEFA decreased drastically when surfactant percentage was increased. However, plastic limit values’ variation was insignificant for CTAC and QEFA Fig.8 and Fig.9.
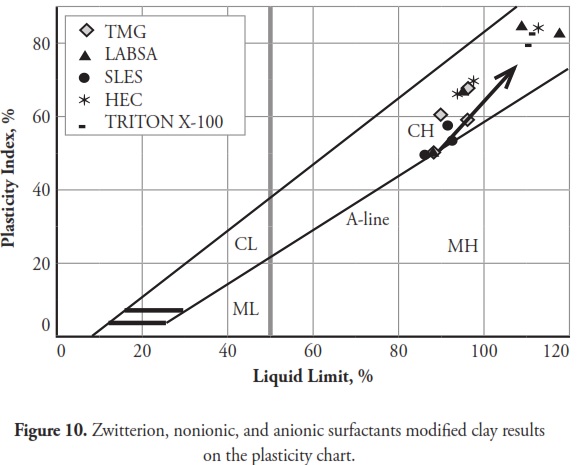
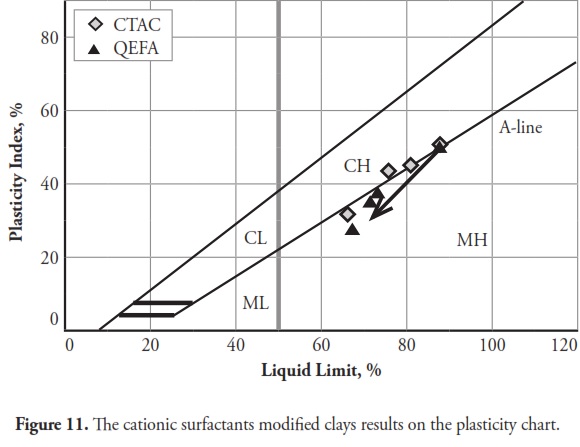
The consistency limit test results were marked on the Cassagrande plasticity chart to determine the new soil classification according to the USCS. Fig 10 shows the changes after modification with anionic surfactants and Fig. 11 shows the changes after modification with cationic surfactants. It can be clearly seen that the points representing soil classification approached the A-line when anionic surfactant percentage was increased. However, cationic surfactants changed the clay class when thepercentage was increased, resulting in cationic SMCs becoming MH (high plasticity silt) Fig. 11. Consistency limit test results would suggest that clay water affinity became significantly increased by LABSA and HEC; however, TMG and SLES did not significantly change water affinity. CTAC and QEFA also decreased water affinity.
It should be pointed out that there has been no general consensus regarding the effect of surfactants and chemicals on clays’ consistency limits. Most researchers have reported that chemicals have decreased the liquid limit of clays (Gleason et al., ., 1997; Shackelford et al., ., 2000; Schmitz et al., ., 2004; Lee et al., ., 2005; Jo et al., ., 2005). However, little research has indicated that chemicals have increased CL or kaolinite clay liquid limit (Rao and Mathew, 1995; Sivapullaiah and Manju, 2005; Park et al., ., 2006; Arasan and Yetimoglu, 2008). Different patterns have most likely arisen from a difference in clay mineralogy and surfactant/chemical type (i.e. zwitterion, anionic, nonionic and/or cationic). In line with previous studies, this research has shown that anionic SMCs were the most hydrophilic and cationic SMCs were the most hydrophobic (Figure 12,Figure 13). Nevertheless, it could be said that the net electrical forces between clay mineral layers were affected by surfactant percentage and type; anionic and cationic surfactants would result in an increase and a decrease in net repulsive forces, respectively (increased and decreased repulsive forces cause dispersion and flocculation of clay particles, respectively).
Contact angle measurements
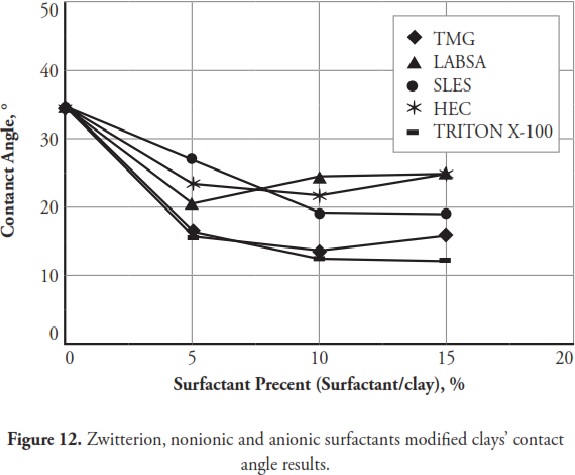
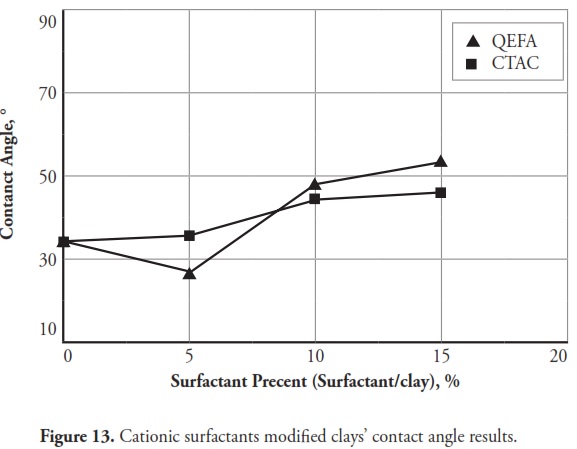
Figure 12 gives the effect of zwitterion, nonionic and anionic surfactants on modified clays’ contact angles and Fig.13 shows the cationic surfactants effect. Figure 14 presents some images of the smallest contact angle obtained from natural and SMCs.
Figure 12shows that zwitterion, nonionic and anionic surfactants significantly decreased modified clays’ contact angles; however, cationic surfactants increased the contact angles (Fig.13) Fig.13. TMG and QEFA were the most effective surfactants when contact angle results were taken into consideration; similar to consistency limit results, contact angle measurements indicated that clay water affinity was increased by zwitterion, nonionic and anionic surfactants and also became decreased by cationic surfactants. Cipriano et al., (2005) indicated that cationic surfactants increased modified clays’ contact angles and produced a hydrophobic surface.
Conclusions
The following conclusions were thus drawn:
Consistency limits were significantly changed compared to those for natural clay. The points representing soil class came further towards the A-line when zwitterion, nonionic and anionic surfactant percentage increased. Cationic surfactants changed the clay classification from CH to high plasticity silt (MH) when the percentage of surfactant added to the clay was increased;
Clays modified with zwitterion, nonionic and anionic surfactants gave the lowest contact angles compared to those for natural clay; however, the clays modified with cationic surfactants gave the highest contact angles; and
It could also be said that clay water affinity was increased by zwitterion (TMG), nonionic (HEC, TRITON X-100) and anionic surfactants (LABSA, SLES), also that cationic surfactants (CTAC and QEFA) decreased the water affinity used in this research. Hence, zwitterion, nonionic and anionic SMCs may be used as hydrophilic materials in waste water remediation and the cationic SMCs may also be used as hydrophobic materials (liner) in waste disposal landfills and dams. It should be pointed out that further studies on SMCs’ engineering properties (e.g. XRD, XRF, DTA and TG for mineralogy and cation exchange capacity, zeta potential for electro-kinetic properties) are needed to make more reasonable judgments. Such studies should aim at explaining SMC behaviour more reasonably.
Acknowledgements
We gratefully acknowledge TUBITAK’s financial support (grant 107Y295).
References
Abdullah, W.S., Alshibli, K.A., Al-Zoubi, M.S., 1999. Influence of Pore Water Chemistry on the Swelling Behavior of Compacted Clays. Applied Clay Science, 15: 447-462. [ Links ]
Akbulut, S., Arasan, S., Acikyildiz, M., 2010. Water affinity of surfactant modified clay. Proceedings of the First Makassar International Conference on Civil Engineering (MICCE2010), ISBN 978-602-95227-0-9. [ Links ]
Alawaji, H.A., 1999. Swell and compressibility characteristics of sandbentonite mixtures inundated with liquids. Applied Clay Science 15,411-430.
Al-Asheh, S., Banat, F., Abu-Aitah, L., 2003. Adsorption of phenol using different types of activated bentonites, Sep. Purif. Technol. 33: 1 10.
Anonymous, 2009. http://en.wikipedia.org/wiki/Contact_angle [ Links ]
Arasan, S., Yetimoglu, T. 2008. Effect of salt solutions on the consistency limits of two clays. Turkish Journal of Engineering and Environmental Sciences, 32: 107-115. [ Links ]
Ashmawy, A.K., El-Hajji, D., Sotelo, N., Muhammad, N., 2002. Hydraulıc performance of untreated and polymer-treated bentonite in inorganic landfill leachates. Clays and Clay Minerals, 50:546-552. [ Links ]
Cipriano, B.H., Raghavan, S.R. McGuiggan, P.M. 2005. Surface tension and contact angle measurements of a hexadecylimidazolium surfactant adsorbed on a clay Surface. Colloids and Surfaces A: Physicochem. Eng. Aspects 262: 8 13. [ Links ]
Dharaiya, D., Jana, S.C., 2005. Thermal decomposition of alkyl ammonium ions and its effects on surface polarity of organically treated nanoclay Polymer, 46(23):10139-10147 [ Links ]
Dolinar, B., Misic, M., Trauner, L., 2007. Correlation between surface area and atterberg limits of fine-grained soils. Clays and Clay Minerals, 55: 519-523. [ Links ]
Fu, X., Qutubuddin, S., 2000. Synthesis of polystyrene clay nanocomposites. Materials Letters, 42:12 15. [ Links ]
Gates, W.P., Nefiodovas, A., Peter, P., 2004. Permeability of an organo-modified bentonite to ethanol-water solutions. Clays and Clay Minerals, 52(2):192-203. [ Links ]
Ghiaci, M., Abbaspur, A., Kia, R., Seyedeyn-Azad, F., 2004. Equilibrium isotherm studies for the sorption of benzene, toluene, and phenol onto organozeolites and assynthesized MCM-41, Sep. Purif. Technol. 40: 217 229. [ Links ]
Gleason, M.H., Daniel, D.E., Eykholt, G.R., 1997. Calcium and Sodium Bentonite for Hydraulic Containment Applications. J. Geotech. Geoenv. Eng. 123(5), 438-445. [ Links ]
Guegan, R., Gautier, M., Beny, J.M., Muller, F., 2009. Adsorptıon of a c10e3 non-ionic surfactant on a ca-smectite. Clays and Clay Minerals, 57: 50 -509. [ Links ]
Gungor, N., Alemdar, A., Atici, O., Ece, I.O., 2001. The effect of SDS surfactant on the flow and zeta potential of bentonite suspensions. Materials Letters, 51: 250 254 [ Links ]
Gurses, A., Karaca, S., Acikyildiz, M., Ejder (Korucu), M., 2009. Thermodynamics and mechanism of cetyltrimethylammonium adsorbtion onto clayey soil from aqueous solutions. Chemical Engineering Journal, 147: 194-201. [ Links ]
He, H., Ding, Z., Zhu, J., Yuan, P., Xi, Y., Yang, D., Frost, R.L., 2005. Thermal characterization of surfactant-modified montmorillonites Clays and Clay Minerals, 53: 287 -293. [ Links ]
Isci, S., Gunister, E., Alemdar, A., Ece, O.I., Gungor, N., 2008. The influence of DTABr surfactant on the electrokinetic and rheological properties of soda-activated bentonite dispersions. Materials Letters, 62:81 84. [ Links ]
Janczuk, B., Chibowski, E., Hajnos, M., Bialopiotrowicz, T., Stawinski, J., 1989. Influence of exchangeable cations on the surface free energy of kaolinite as determined from contact Angles. Clays and Clay Minerals, 37: 269-272. [ Links ]
Jefferson, I., Rogers, C.D.F., 1998. Liquid Limit and the Temperature Sensitivity of Clays. Engineering Geology, 4(9), 95-109. [ Links ]
Jo, H.Y., Benson, C.H., Shackelford, C.D., Lee, J.M., Edil, T.B., 2005. Long-Term Hydraulic Conductivity of A Geosynthetic Clay Liner Permeated with Inorganic Salt Solutions. J. Geotech. Geoenv. Eng. 131(4),405-417. [ Links ]
Jouany, C., Chassin, P., 1987. Determination of the surface energy of clay-organic complexes by contact angle measurements. Colloids Surface, 27 (4): 289 303. [ Links ]
Kalkan E, Bayraktutan MS (2008) Geotechnical evaluation of Turkish clay deposits: a case study in Northern Turkey. Environmental Geology, 55:937 950. [ Links ]
Lee, J.M., Shackelford, C.D., Benson, C.H., Jo, H.Y., Edil, T.B., 2005. Correlating Index Properties and Hydraulic Conductivity of Geosynthetic Clay Liners. J. Geotech. Geoenv. Eng., 131(11), 1319-1329. [ Links ]
Li, Z., Willms, C.A., Kniola, K., 2003. Removal of anionic contaminants using surfactant-modified palygorskite and sepiolite. Clays and Clay Minerals, 51: 445-451. [ Links ]
Liu, R., Frost, R.L., Martens, W.N., Yuan, Y., 2008. Synthesis, characterization of mono, di and tri alkyl surfactant intercalated Wyoming montmorillonite for the removal of phenol from aqueous systems. Journal of Colloid and Interface Science, 32(2): 287-294. [ Links ]
Lo, I.M.C., 2001. Organoclaywith soil bentonite admixture aswaste contaminant barriers, J. Environ. Eng. 127: 154 161. [ Links ]
López-Durán, J.D.G., Khaldoun, A., Kerkeb, M.L., Ramos-Tejada, M.M., González-Caballero, F., 2003. Wettability of montmorillonite clays in humic acid solutions. Clays and Clay Minerals, 51: 65-74. [ Links ]
Matott, L.S., Bartelt-Hunt, S.L., Rabideau, A.J., Fowler, K.R., 2006. Application of heuristic optimization techniques and algorithm tuning to multilayered sorptive barrier design, Environ. Sci. Technol. 40: 6354 6360. [ Links ]
Met, I., Akgun, H., Turkmenoglu, A.G., 2005. Environmental geological and geotechnical investigations related to the potential use of Ankara clay as a compacted landfill liner material, Turkey. Environmental Geology 47, 225-236. [ Links ]
Mitchell, J.K., Soga, K., 2005. Fundamentals of Soil Behavior, 3rd Edition, John Wiley & Sons, Inc., Hoboken, New Jersey, p 577. [ Links ]
Mulligan, C.N., Yong, R.N. and Gibbs, B.F., 1999a. On the use of biosurfactants fort he removal of heavy metals from oil-contaminated soil. Environ. Prog. 18: 31-35. [ Links ]
Mulligan, C.N., Yong, R.N. and Gibbs, B.F., 1999b. Removal of heavy metals from contaminated soil and sediments using the biosurfactant surfactin. J. Soil Contam. 8: 231-254. [ Links ]
Mulligan, C.N., Yong, R.N. and Gibbs, B.F., 2001. Surfactant-enhanced remediation of contaminated soil: a review. Engineering Geology. 60: 371-380. [ Links ]
Park, J., Vipulanandan, C., Kim, J.W., Oh, M.H., 2006. Effects of surfactants and electrolyte solutions on the properties of soil. Eviron Geol. 49: 977-989. [ Links ]
Rao, S. N. and Mathew, P. K., 1995. Effects of exchangeable cations on hydraulic conductivity of a marine clay. Clays and Clay Minerals, 43(4), 433-437. [ Links ]
Rogers K., Takacs E., Thompson M.R., 2005. Contact angle measurement of select compatibilizers for polymer-silicate layer nanocomposites. Polymer Testing, 24 (4): 423-427. [ Links ]
Schmitz, R.M., Schroeder, C., Charlier, R.,2004. Chemo-Mechanical Interactions in Clay: a Correlation Between Clay Mineralogy and Atterberg Limits. Applied Clay Science, 26,351-358. [ Links ]
Shackelford, C.D., Benson, C.H., Katsumi, T., Edil, T.B., Lin, L., 2000. Evaluating the Hydraulic Conductivity of GCLs Permeated with Non-Standart Liquids. Geotextiles and Geomembranes, 18,133- 161. [ Links ]
Sharma, H.D., Lewis, S.P., 1994. Waste Containment Systems, Waste Stabilization, and Landfills: Design and Evaluation. John Wiley & Sons Inc., Canada, pp.588. [ Links ]
Sivapullaiah, P.V., Manju, -Kaolinite-Alkali Interaction and Effects on Basic Properties-, Geotechnical and Geological Engineering, 23,601-614, 2005. [ Links ]
Wibulswas, R., 2004. Batch and fixed bed sorption of methylene blue on precursor and QACs modified montmorillonite, Sep. Purif. Technol. 39: 3 12. [ Links ]
Xi, Y., Zhou, Q., Frost, R.L., He, H., 2007. Thermal stability of octadecyltrimethylammonium bromide modified montmorillonite organoclay. Journal of Colloid and Interface Science, 311(2):347-353. [ Links ]
Yang, J.W., Lee, Y.J., Park, J.Y., Kim, S.J., Lee, J.Y., 2005. Application of APG and Calfax 16L-35 on surfactant-enhanced electrokinetic removal of phenanthrene from kaolinite. Engineering Geology, 77: 243 251. [ Links ]
Yilmaz, G., Yetimoglu, T., Arasan, S., 2008. Hydraulic Conductivity of Compacted Clay Liners Permeated with Inorganic Salt Solutions. Waste Management & Research, 26: 464-473. [ Links ]
Yukselen, Y., Kaya, A., 2006. Prediction of cation exchange capacity from soil index properties. Clay Minerals, 41: 827-837 [ Links ]
Zhu, L., Li, Y., Zhang, J., 1997. Sorption of organo-bentonites to some organic pollutants in water. Environ. Sci. Technol. 31: 1407 1410. [ Links ]