Rabies virus
The rabies virus (RABV) is an RNA virus that belongs to the order Mononegavirales1, genus Lyssavirus, and in the family Rhabdoviridae2. The virus particles are bullet shaped and measure 75 nm in diameter with a length of 200 nm 3. The genus Lyssavirus includes fourteen species which are classified by their genomic sequence; they are the RABV, Lagos bat virus (LBV), Mokola virus (MOKV), Duvenhage virus (DUVV), European bat lyssavirus type 1 (EBLV1), European bat lyssavirus type 2 (EBLV2), Australian bat lyssavirus (ABLV), Aravan virus (ARAV) and Khujand virus (KHUV), Irkut virus (IRKV), West Caucasian bat virus (WCBV), Bokeloh bat lyssavirus (BBLV), Ikoma Lyssavirus (IKOV) and Shimoni bat virus (SHIBV). Nine of the fourteen species of lyssavirus (DUVV, EBLV1, EBLV2, ABLV, ARAV, KHUV, IRKV, WCBV and BBLV) have been isolated from insectivorous bats. Additionally, the members of the genus Lyssavirus were subdivided into three phylogroups (I, II and III). Phylogroup I includes RABV, DUVV, EBLV1, EBLV2, ABLV, ARAV, KHUV, IRKV and BBLV (2,4. Phylogroup II includes LBV and MOKV and a new lyssavirus named “Shimoni bat virus” (SHIBV), which was isolated from a freshly dead insectivorous bat (Hipposideros commersoni) in the coastal region of Kenya in 2009 and was found to belong to the Phylogroup II lyssaviruses5. Phylogroup III is compounded by the WCBV and one currently unclassified lyssaviruses identified as IKOV2. The RABV genome consists of an approximately 12 kb nonsegmented, single negative strand RNA molecule 6, encoding five structural proteins that are: the nucleocapsid (N) protein, a phosphoprotein (P), matrix (M) protein, glycoprotein (G), and an RNA-dependent RNA polymerase (L) in the order 3’-N-P-M-G-L-5’ 7. It is impossible to make a clinical differentiation between the disease caused by any virus species 8.
Rabies, the disease
Rabies is one of the most important zoonotic diseases that is caused by a highly neurotropic Rhabdovirus9. The disease is reported in domestic and wild animals worldwide and it’s estimated to cause up to 70,000 human deaths per year, mostly in rural areas of Asia and Africa 10. Rabies represents a neuroinvasive disease that is characterized by acute encephalitis with two clinical manifestations; the furious (classical or encephalitic) and the paralytic form. Furious rabies is the most common form of human rabies, accounting for approximately 80% of cases. With the exception of Antarctica, rabies is endemic to all continents. Classical RABVs have a worldwide distribution except for a few island nations such as Great Britain, Ireland, New Zealand, Hawaii, the continents of Australia and Antarctica, and an increasing number of Western European countries 11. The disease affects a broad spectrum of warm blooded animals. All mammals are susceptible to varying degrees, especially members of the order Carnivora and Chiroptera10.
Rabies is an invariable fatal disease, particularly when clinical symptoms have developed. Rabid animals commonly show neurological changes such as paraparesis, aggressiveness, hydrophobia and sialorrhea, agitation, mental confusion, and tetraparesis 12. In Latin America, rabies is classified into two epidemiologic forms; urban rabies and sylvatic rabies. The common vampire bat, Desmodus rotundus, has emerged as the principal RABV reservoir host along the species natural range from Mexico to South America. D. rotundus was correlated with the vampire bat RABV variants which is more prevalent than the dog RABV variants in Peru, however, the prevalence of any RABV variant might be dependent of local or regional RABV vaccination plans 9.
Pathogenesis of rabies
Paralytic rabies is characterized by flaccid paralysis in the bitten limb, which ascends symmetrically or asymmetrically, whereas the furious rabies manifests hyper-excitability, autonomic dysfunction, hydrophobia, and aerophobia 13,14.
RABV may enter the organism by different transmission routes such as animal bites or scratches, and the virus can remain latent close to the inoculation site for long periods of time. The virus replicates slowly within muscle cells until it arrives to the neuromuscular junction 14. The viral glycoprotein is essential for transsynaptic spreading by using cell nicotinic acetylcholine receptors at the neuromuscular junctions 15. The virus migrates along peripheral nerves to the central nervous system via retrograde fast axonal transport, a process that is facilitated by the viral P protein 17, at a rate of 12 to 100 mm/d16. In the CNS, RABV replicates in neurons and induces necrosis and inflammation18. Then, the virus spread to other organs of the body, reaching the salivary gland where high viral concentrations of virus could be found in the saliva, peripheral nerves and other organs 7 (Figure 1).
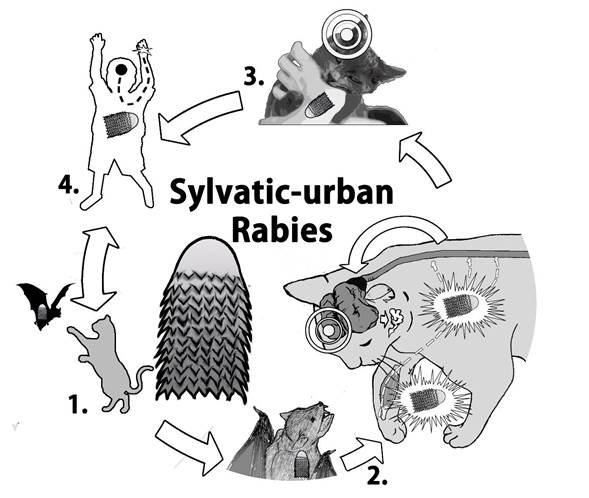
Figure 1 Connection of sylvatic rabies to urban ecosystem. 1. The cat hunts an infected chiropter. 2. The cat is bitten, the virus enter to the body through the wound by infiltrating of saliva, and the virus replicates in the peripheral muscles close to the wound and migrates to CNS. In the CNS, the virus is distributed to other organs, eventually reaching the salivary glands. 3. The rabid animal shows neurological changes. 4. The animal is predisposed to attack humans or other animals. The cycle repeats and causes the death of the infected individual.
RABV might be present in different tissues of bats: particularly the buccal cavity, saliva, and brown fat. The distribution of RABV in tissues was evaluated in 26 bat species from Brazil. The species Artibeus lituratus (13), Myotis nigricans (4), Eptesicus furinalis (5), Eptesicus diminutus (1), Lasiurus blossevillii (1), and Lasiurus ega (2) were tested by using hnRT-PCR. The virus was detected in tongue (92% and 85%), brown fat (82% and 77%), lung (62% and 77%), heart (42% and 77%), stomach (92% and 64%), liver (38% and 67%), spleen (43% and 27%), bladder (73% and 88%), kidney (77% and 38%), intestine tissues (77% and 38%), and feces (38% and 42%) from frugivorous and insectivorous bats. It was noted that the virus was higher in stomachs of frugivorous bats than from insectivorous bats 19. A possible reason for those findings could be that infected bats defecate on the fruits that are eaten later by frugivorous bats, which ingest the virus. Contrary, insectivorous bats, due to their eating habits, may have lower viral loads, However, additional studies are necessary to determine the relationship between feeding habits of the non-hematophagous bats and the rabies virus.
Rabies virus transmission
The RABV is usually transmitted from an infected animal to one that’s susceptible 1. The virus is usually present in the saliva of rabid animals, and it enters the body via infiltration of virus-laden saliva into a wound or by the exposure of mucosal surfaces to saliva from an infected animal (bites) 20. Additionally, a rare form of transmission may be by the inhalation of aerosols with RABV present. This would most likely occur in caves with dense populations of bats in which the virus is present 21.
The RABV and the majority of lyssaviruses, are found in natural bat reservoirs. These animals are unique among mammals due to having exceptional sociality and longevity. Given these features and the recognized status of bats as reservoirs for RABVs in the Americas, individual bats may experience repeated exposure to RABV during their lifetime 22. Hematophagous, frugivorous, and insectivorous bats can transmit the RABV 23, and phylogenetic analysis using nucleotide sequences of N or G genes revealed that RABV is grouped into clusters according to the bat species that support the existence of species-specific variants or lineages of the virus 19,24.
Main species of Bats reservoirs for rabies virus in the world
The class Mammalia has 5,416 species and the order Chiroptera comprises of the second largest group of mammals in number, with 1,120 species 23. Bats have a worldwide distribution, absent only in the Polar Regions and some oceanic islands. Most of these animals live in tropical and subtropical regions, but can be found in temperate regions 25. A number of bats reservoirs for RABV has been described, including hematophagous, insectivorous and frugivorous bats (Table 1), although the RABV has been detected mainly in insectivorous bat species (Figure 2).
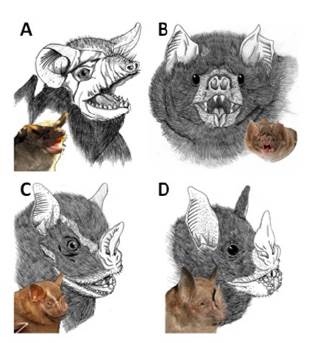
Figure 2 Main bat reservoirs of RABV are represented by a photograph and a schematic diagram. A. Tadarida brasiliensis (insectivoro), B Desmodus rotundus (hematophagous), C Artibeus lituratus (frugivorous) and D. Carollia perspicillata (frugivorous).
Insectivorous bat species have worldwide distribution and can be found in almost all ecosystems, together with others frugivorous bats species, whereas all the three species of hematophagous bats are only found in Latin America, with the common vampire (Desmodus rotundus) being the only well-known RABV reservoir 26.
Table 1 Chiroptera species that have been reported positive for the presence of RABV in the world.
| N | Species | Location | Ecology | Reference |
|---|---|---|---|---|
| 1 | Desmodus rotundus | Brazil/Colombia | hematophagous | 12, 27 |
| 2 | Diphylla ecaudata | Brazil | hematophagous | 8, 25 |
| 3 | Diaemus youngi | Brazil | hematophagous | 25 |
| 4 | Tadarida brasiliensis | Chile | insectivorous | 8, 28 |
| 5 | Lasiurus cinereus | Chile | insectivorous | 28 |
| 6 | Histiotus macrotes | Chile | insectivorous | 28 |
| 7 | Myotis chiloensis | Chile | insectivorous | 28 |
| 8 | Artibeus lituratus | Brazil | frugivorous | 24 |
| 9 | Chrotopterus auritus | Brazil | omnivorous | 25 |
| 10 | Eptesicus fuscus | United state | insectivorous | 22 |
| 11 | Myotis sp | United state | insectivorous | 29 |
| 12 | Lasionycteris noctivagans | United states migratory tree-roosting hoary | insectivorous | 30 |
| 13 | Lasiurus cinereus | United states migratory tree-roosting hoary NA | insectivorous | 30 |
| 14 | Eptesicus spp | Brazil | insectivorous | 31 |
| 15 | Nyctinomops macrotis | Brazil | insectivorous | 25 |
| 16 | Molossus spp | Brazil | insectivorous | 31 |
| 17 | Tadarida spp | Brazil | insectivorous | 31 |
| 18 | Histiotus velatus | Brazil | insectivorous | 25 |
| 19 | Lasiurus spp | Brazil | insectivorous | 31 |
| 20 | Molossus ater | Brazil | insectivorous | 32 |
| 21 | Molossus molossus | Brazil | insectivorous | 33 |
| 22 | Eumops auripendulus | Brazil- Ecuador | insectivorous | 33 |
| 23 | Nyctinomops laticaudatus | Brazil- Ecuador | insectivorous | 33 |
| 24 | Eptesicus furinalis | Brazil- Ecuador | insectivorous | 33 |
| 25 | Lasiurus cinereus | United states | insectivorous | 34 |
| 26 | Lasiurus borealis | United states/ Chile | insectivorous | 28, 34 |
| 27 | Lasionycteris noctivagans | United states | insectivorous | 35 |
| 28 | Dasypterus floridanus | Florida/ solitary | insectivorous | 35 |
| 29 | Phyllostomus supercilliatum | United states | insectivorous | 36 |
| 30 | Myotis evo | United states | insectivorous | 35 |
| 31 | Antrozous pallidus | Canada, México and Cuba | insectivorous | 35 |
| 32 | Macrotus californicus | Mexico and the United States | insectivorous | 35 |
| 33 | Pipistrellus hesperus | western United States and Mexico | insectivorous | 35 |
| 34 | Myotis keenii | Canada | insectivorous | 37 |
| 35 | Euderma maculata | Canada | insectivorous | 37 |
| 36 | Myotis lucifugus | Canada | insectivorous | 37 |
| 37 | Myotis yumanensis | Canada | insectivorous | 37 |
| 38 | Lasiurus intermedius | Canada | insectivorous | 37 |
| 39 | Myotis evotis | Canada | insectivorous | 37 |
| 40 | Myotis nattereri | German and France | insectivorous | 38 |
| 41 | Myotis dasycneme | Germany | insectivorous | 38 |
| 42 | Myotis daubentonii | Germany | insectivorous | 38 |
| 43 | Eptesicus isabellinus | Germany | insectivorous | 38 |
| 44 | Eptesicus serotinus | Germany- Spain | insectivorous | 38, 39 |
| 45 | Pipistrellus nathusii | Germany | insectivorous | 38 |
| 46 | Miniopterus schreibersii | Southeastern Europe | insectivorous | 40 |
| 47 | Hipposideros commersoni | Kenya | insectivorous | 5 |
| 48 | Rousettus aegyptiacus | Europe Mediterranean | frugivorous | 5 |
| 49 | Pipistrellus pipistrellus | Spain EBLV1, EBLV2 | insectivorous | 39 |
| 50 | Artibeus jamaicensis | Kenia | frugivorous | 8 |
| 51 | Eidolon helvum | Nigeria | frugivorous | 41 |
| 52 | Rousettus aegyptiacus | Kenia | frugivorous | 41 |
| 53 | Micropteropus pusillus | Central African Republic | frugivorous | 41 |
| 54 | Epomophorus wahlbergi | South Africa | frugivorous | 41 |
| 55 | Nycteris gambiensis | Guinea | insectivorous | 42 |
| 56 | Artibeus obscurus | Brazil | frugivorous | 25 |
| 57 | Phyllostomus hastatus hastatus | Brazil | insectivorous | 25, 43 |
| 58 | Uroderma bilobatum | Colombia | insectivorous | 44 |
| 59 | Phyllostomus hastatus | Colombia | Frugivoro- insectivorous45 | 44 |
| 60 | Eptesicus braziliensis | Colombia | insectivorous | 46 |
| 61 | Carollia perspicillata | Colombia | frugivorous | 47 |
| 62 | Myotis nigricans | Colombia | insectivorous | 47 |
| 63 | Lasiurus ega | Colombia | insectivorous | 47 |
| 64 | Molossus molossus | Colombia | insectivorous | 48 |
Rabies cases and virus variants in Colombia
RABV in Colombia have been grouped into three variants, Colombian genetic variant I viruses (isolated in Arauca and the Central Region of the country), Colombian genetic variant II viruses (isolated in the Caribbean Region) and the third group that consists of viruses isolated from two insectivorous bats (Eptesicus brasiliensis and Molossus molossus), three domestic dogs and a human. The genetic sequence analysis indicated that the virus isolates belonging to the third group were variants of bat RABV, the first finding that associated bats to rabies in dogs and humans in Colombia 49. Rabies in Colombia and other Latin American countries is an important public health and economic problem, and the disease is categorized as urban rabies and sylvatic rabies, which have distinct epidemiological cycles 50,51. Urban rabies is usually transmitted by the domestic dog and this animal is the main transmitter and reservoir of the virus, whereas sylvatic rabies is transmitted principally by bats the main reservoirs of the disease that is transmitted to domestic animals such as cows and horses, however, mongooses and coyotes can share the Variant 1(urban rabies) with domestics dogs in less proportion 49,56.
Wild RABV variants identified in Colombia are V3 (hematophagous bats), V4 (insectivorous bats), V5 (hematophagous bats) and V8 (skunk) 52. A study that analyzed a total of 124 samples obtained from human cases of rabies and 8 from other mammal species within the period 1994-2005, identified eight genetic lineages (GL1- GL8), of RABV. Phylogenetic analyses of the partial nucleoprotein gene sequence determined specific variants within those genotypes. The GL4 comprised of Variant V3 and V8 and a variant no determined (ND), which were associated with hematophagous bats, the GL5 and GL6 consisted of V4 viruses associated with Tadarida brasiliensis bats, the GL5 grouped independently. The GL7 and GL8 segregated independently within clades associated with colonial insectivorous and solitary bats, both of these were no determined variants. RABVs isolated from humans grouped within GL2, GL3 and GL4, which corresponded to V1, V3, V8 and ND. Dogs and Desmodus rotundus are the two major RABV reservoirs and vectors in Colombia, although insectivorous bats may also be involved 53.
RABV variants V3 and V4 are the most prevalent in Colombia, and this situation seems to be influenced by increased deforestation and urban architecture that provides shelter, causing more frequent interactions between humans and bats 44. The variant V4, that is associated with frugivorous and insectivorous bats, was isolated from a dog and a human in the northern of Colombia 54.
RABV was isolated for the first time from bats in Colombia by Alarcon in 1968, who reported the isolation of two strain of RABV from insectivorous and frugivorous bats (Myotis nigricans, Lasiurus ega and Carollia perspicillata) captured from areas of the departments of Guajira, Santander and Antioquia. Other bat species such as Glossophaga longirostris, Artibeus lituratus palmarum, Platyrrhinus Helleri (Vampyrops Helleri), Trachops Cirrhosus cirrhosus, Peroptexys kappleri, Phyllostomus Hastatus, Saccopteryx bilineata, and Molossus molossus were negative to the virus 47. In the urban zone of Cali, the species Carollia perspicillata, Artibeus lituratus, Eptesicus brasiliensis, Myotis nigricans, Molossus molossus, and Tadarida brasiliensis were also reported as transmitters of RABV in the period December 2000 to June 2002 55.
The National Institute of Health of Colombia has reported a considerable number of cases of sylvatic rabies transmitted by bats since 2000. A total of thirty-five cases of human rabies, twenty-two of those cases were transmitted by bats, eight by cats, and five by dogs. Regarding the virus variants, two were variant VA (atypical), five- Variant V1 (domestic and wild dogs, mongooses and coyotes), twenty-four-variant V3 (hematophagous bat), three-Variant V4 (insectivorous bat), and one of those cases was variant V8 (skunk) 56. Outbreaks of rabies disease have been recorded in high magnitude in Bajo Baudo, Choco, where hematophagous bats represent the major threat for sylvatic rabies transmission 51,57. Outbreaks of rabies caused by variants V3 have also been recorded in San Luis de Palenque in Casanare and Floridablanca in Santander 56, and Santander de Quilichao in Cauca, where a cat was the transmitter to humans, linking sylvatic rabies and the urban ecosystem 27. In Encino and Piedecuesta in Santander, other two human rabies cases were reported to be caused by the variant V3 transmitted by cat and a hematophagous bat, respectively. Cats usually get infected by contact with bats during their predatory behavior 50.
Outbreaks of rabies caused by variant V4 were reported in Moniquirá, Boyacá, where the insectivorous bat Tadarida brasiliensis was identified as the main reservoir 44. The variant V4 was also responsible for an outbreak in Roldanillo, Valle del Cauca in 2012 56, whereas in Barrancabermeja, Santander an outbreak was caused by the atypical variant VA 56. An atypical RABV variant from sylvatic origin was also responsible for the disease in a child that had contact with a cat in San Luis, Tolima in 2010 (INS, 2014).
Diagnosis of rabies infection
The diagnosis of the virus is conducted by the use of a number of methodologies that include the detection of rabies antigens in tissues, nucleic acids, amplification of rabies particles or serological tests.
Mouse inoculation test
It consist of an intracerebral inoculation of a clarified supernatant of a 10-20 % (w/v) homogenate of brain material including brainstem (cortex, Ammon’s horn, thalamus, medulla oblongata) in an isotonic buffered solution with antibiotics, into groups of 3 to 10 mice that should be observed by 28 days to record mortality and detection of RABV. In the case of newborn mouse, they can be evaluated on days 5, 7, 9 and 11 post-inoculation. Any deaths occurring during the first 4 days are regarded as nonspecific (due to stress or bacterial infection) 59.
Fluorescent antibody test (FAT)
It is the most widely used test for rabies diagnosis recommended by WHO and OIE as the gold standard test and it may be used directly on a smear. The test uses purified immunoglobulin previously conjugated with fluorescein isothiocyanate (FITC) that is added onto an acetone-fixed brain tissue smear, preferably made from several parts of the central nervous system. FAT provides a reliable diagnosis in 98-100% of cases for all RABV strains if a potent conjugate is used. FAT can be applied in fresh or frozen brain tissues sections, with very similar results (99.8% sensitivity and 100% specificity) when applied to fresh or formalin-fixed tissues 61, however, it should not be used in decomposed tissue samples 60.
Reverse transcription PCR (RT-PCR)
Classical reverse transcription-polymerase chain reaction assay has been reported to be a sensitive and specific tool for routine diagnostic purposes 62. It consist of the use of a reverse transcriptase to synthesize a complementary DNA copy from viral RNA and then, conventional PCR is used to amplify a gene fragment from the virus genome 63. The technique can be used to detect RABV in decomposed samples that often appear due to the warm climate and fluctuations in ambient temperature during sample transport and storage 64. Positive diagnostic results from such samples are reliable but negative results may be invalid 58.
A comparison of RT-PCR and MIT for detection of RABV in 95 positive samples that were stored for 4-13 years at −20 and −80 °C revealed that only 32 (33.6%) of the samples were positive with the mouse inoculation test, while RT-PCR detected the viral genome in 62 (65,3%) samples. Samples that were stored for >10 years gave 59.7% positivity by RT-PCR and only 22.1% by MIT 65.
Heminested RT-PCR (HnRT-PCR): It is one of the most sensitive and rapid technique for rabies diagnosis. The method can be applied to both living animals and post mortem collected samples, when the brain samples are in a decomposed state. The PCR products can be used for DNA sequencing for final identification of virus origin by epidemiological analysis 66. Our group implemented this technique to analyze brain tissues from a number of bats collected in rural areas of the Tolima region (Figure 2). A total of eleven bats species including Artibeus jamaicensis, Artibeus lituratus, Carollia perspicillata, Desmodus rotundus, Molossus molossus, Molussus ater, Myotis nigricans, Phyllostomus hastatus, Platyrrhinus dorsalis, Saccopteryx bilineata and Saccopteryx leptura were analyzed by HnRT-PCR and all samples were negative for the presence of RABV (Figure 2).
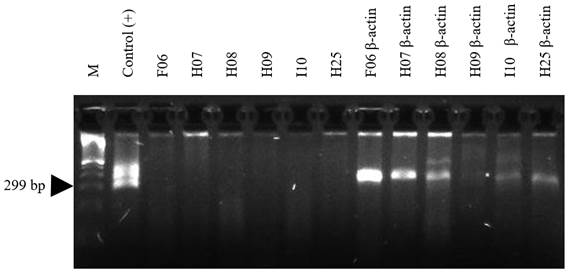
Figure 3 HnRT-PCR amplification of 299 bp of RABV N gene from chiropters brain tissues. Total RNA was extracted from bran tissue of six captured bats in the Tolima region and subjected to RT-PCR analysis and the cDNA construction was validated with the amplification of the housekeeping gene b-actin in each sample. M: 100 bp DNA ladder; Positive control RNA from RABV was kindly provided by Dr. Andres Páez from the National Institute of Health of Colombia (INS). F06, H07, H08, H09, I10 and H25 corresponds to brain bats samples.
Real time PCR.
It is an alternative test that have been demonstrated to be more sensitive than HnRT-PCR and can be used to further extend the rabies RNA detection limits in decomposed samples 67. This technique allows to assess gene expression analysis, determination of viral load and detection of genetically modified organisms 68. The primers are designed in such a way that a fluorescent signal is generated only when the primers are incorporated into an amplification product. Detection of target sequences occurs by monitoring the fluorescence generated by intercalating dyes or fluorophore labelled primers for sequence-specific probes 69. This assay has high sensitivity and specificity enabling simultaneous amplification and quantification of specific nucleic acid sequences that made it exceptional for diagnosis for this infectious agent 69,70. Real time PCR was compared to conventional RT-PCR to analyze saliva samples from 21 suspected patients and found that the sensitivity was superior (75% vs. 37%) to that offered by RT-PCR 71.
Enzyme linked immunosorbent assay ( ELISA )
The ELISA is the most used diagnostic test for rabies, which measures specific immunoglobulins such as IgM. The test consist in the detection of RABV neutralizing antibody in sera samples taken from the suspected animal 72. The test reduce time, facilitate handling and avoid the use of biosecurity level 2 or 3 laboratories, do not require live RABV or cell culture and can be automated. The test was developed for domestic carnivores and wildlife and is the only one certified and prescribed by the OIE for rabies detection. However, the ELISA test, although it may have 100% specificity, the sensitivity was around 78.2% when 593 samples of domestic carnivores were evaluated 73,74.
Prevention of rabies
Rabies is considered a vaccine-preventable disease and annual vaccination of pets is the recommended strategy to prevent and control rabies 76). However, it has been reported that epizootic areas of the rabies virus are self-limiting and D. rotundus sacrificial campaigns have minimal or no incidence on the rate of presentation of the disease in these areas (77. In the case of an attack by a potentially rabid animal, it must be immediately informed to the competent health authorities to start an appropriate research (study of focus, rabies vaccination of dogs and cats, watching suspected animals, taking and sending samples, and institutional active search) and specific treatment which is contemplated by the INS 52 . It is recommended to avoid wild animals as pets and not to handle animals suspected of carrying the disease (dogs, cats, cattle) or handle bats that are on the ground or showing abnormal behavior 75. Competent authorities in each country must have an active surveillance system for the disease and appropriate vaccination programs in areas with high risk of presentation 10. Unfortunately, in Colombia the coverage of vaccination of pets is very limited, the percentage of vaccination coverage of municipalities in Colombia is limited only 59,9% of municipalities have a % coverage ≥ 80%, 7,96% of municipalities a % coverage between 70 and 79%, 6,64% of the municipalities a % coverage between 60-69% and 25,5% of the remaining municipalities a % coverage ≤ 59% 78.
Conclusions
Rabies is a zoonotic disease caused by a neurotropic virus that is present in many species of chiropters all over the world. RABV induces an acute encephalitis, clinically manifested as furious and a paralytic form. The virus is classified in Latin countries as urban rabies and sylvatic rabies that are transmitted by domestic animals and bats respectively (Figure 1). Insectivorous and frugivorous bats act predominantly as reservoirs of rabies and transmitters of the disease in many part of the world. In Colombia, variants V3,V4 and VA (variant hematophagous bat, variant insectivorous bat and variant atypical associated with bats), of the virus, are responsible for a significant number of rabies outbreaks in human 52. The disease is mainly linked to felines such as cats that may hunt infected bats, getting infected too, which make a rabid cat that usually contact with humans, transmitting the virus. Thus evaluation of rapid diagnosis techniques such as RT-PCR to detect RABV in bat tissues might be needed to promote active surveillance to bats populations. Rabies is prevented by annual vaccination of pets, however, the coverage of this activity in Colombia is still limited, thus much effort is needed to educate and sensitize the people on this fatal disease.














