INTRODUCTION
Super-refractory status epilepticus (SRSE) is a pathology, with significant morbidity and mortality rates 1, that affects the neuronal environment depending on the types of seizure and their duration 2. Generally, its causes are divided into genetic and symptomatic status epilepticus (SE); the latter, in turn, is divided into acute and remote and may be caused by structural alterations or metabolic or immunological disorders. The last category includes Febrile Infection-Related Epilepsy Syndrome (FIRES), an encephalopathy characterized by multifocal and refractory SE that occurs in previously healthy children or adolescents.
The onset of SRSE is acute and, despite a usual history of febrile symptoms, the cerebrospinal fluid (CSF) does not present alterations or infectious focus documented frequently 3. Initially, isolated seizures, which quickly evolve to SE, may be observed 4. This paper presents a FIRES case that describes the diagnostic and therapeutic challenges posed by this condition, as well as its management difficulties in the long-term and its consequences on quality of life.
CASE PRESENTATION
7-year-old male patient from Bogotá, Colombia, without relevant family, psychosocial, prenatal or perinatal or pathological history and normal neurodevelopment. The child consulted due to severe headache of 8 days of evolution after a febrile episode managed with cephalexin, considering suspicion of unconfirmed sinusitis. Subsequently, seizures appeared in the following 48 hours, characterized by cephalic and labial version to the left and clonic generalization lasting 1-2 minutes. The seizures became more frequent until consciousness deteriorated and motor status epilepticus appeared. In consequence, management with barbiturate coma was initiated in the ICU, where multifocal clonic and myoclonic seizures continued. Different anticonvulsant treatments were used for 6 weeks: thiopental sodium, lidocaine, propofol, midazolam, valproic acid, topiramate, carbamazepine, phenytoin and clonazepam; the last 6 antiepileptic drugs were used simultaneously and at maximum recommended doses, achieving a poor initial response, but eventually leading to total crisis control.
Possible infectious, inflammatory, metabolic, autoimmune and toxicological etiologies were discarded, while initial neuroimages did not show any alteration (Table 1).
Table 1 Paraclinical tests of the patient
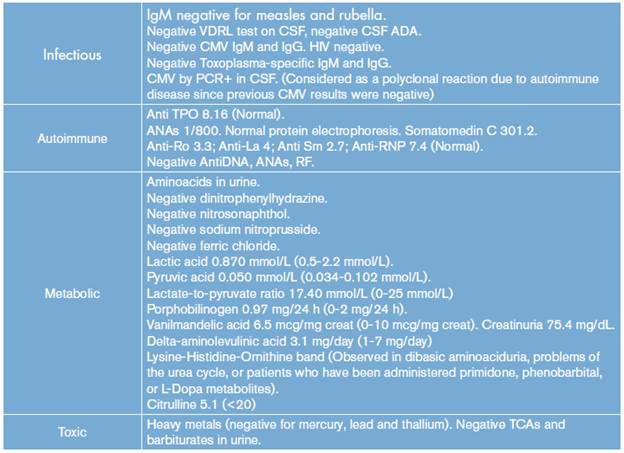
CMV: cytomegalovirus; ADA: adenosine deaminase; anti-TPO: anti thyroid peroxidase antibody; Anti-Sm: anti-Smith antibody; anti-RNP: anti-ribonucleoprotein antibody; ANAs: antinuclear antibodies; RF: rheumatoid factor; TCA: tricyclic antidepressants.
Source: Own elaboration based on the data obtained in the study.
After the initial symptoms resolved, the patient developed a focal drug-resistant epilepsy, with recurrent status epilepticus. Currently, after a 12-year follow-up and treatment with oxcarbazepine, phenobarbital, valproic acid, topiramate, levetiracetam and clobazam in different associations and at maximum tolerated doses, the patient presented with focal ictal frequency of 2 to 3 events per week and altered consciousness. Many of these episodes were associated with bradycardia, decline in oxygen saturation and a monthly event with tonic-clonic generalization, as well as moderate cognitive deficit.
At the time of the case analysis, lacos-amide was added to the topiramate, clobazam and levetiracetam scheme in association with immunoglobulin, which was administered for 6 months, and a low glycemic ketogenic diet (KD). This scheme did not help to decrease ictal frequency, but his relatives reported cognitive improvement with the use of KD.
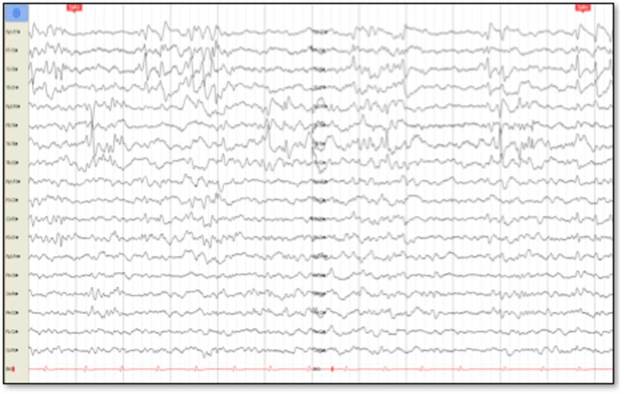
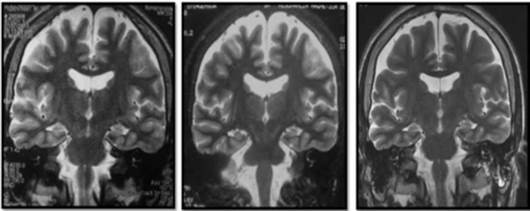
Cerebral nuclear magnetic resonance (NMR) showed generalized cortical atrophy and bilateral hippocampal atrophy (Figure 1), while electroencephalogram revealed independent bilateral temporal ictal activity, frequently associated with bradycardia and hypoxemia (Figure 2).
Source: Own elaboration based on the data obtained in the study.
Figure 1 Simple cerebral magnetic resonance, T2 sequence. From left to right: 2008 (11 years old), 2011 (14 years old), 2016 (19 years old).
DISCUSSION
Etiology
The temporary correlation between fever and the onset of SE has led to consider an autoimmune origin for this syndrome, although current evidence does not confirm this suspicion.
In autoimmune epilepsies, epileptic seizures relate to the production of autoantibodies against central nervous system (CNS) molecules, which is confirmed when some patients respond to immunomodulatory therapy 5,6 and when some autoimmune diseases have a higher crisis incidence 7.
Innate and adaptive immunity has been observed in patients with autoimmune epilepsies, which increases inflammatory mediators, macrophages and neutrophils, activating glial cells and stimulating neuronal death. Mediators seem to act as excitatory agonists, generating a postictal state 4,8-11. Autoanti-bodies have been proposed as the etiology of FIRES, without finding a direct causal association in all cases or being clear whether autoimmune inflammatory processes arise first or if inflammatory activity is the result of the epileptic event 3,11. However, evidence does not support this hypothesis completely given the variability of antineuronal antibody findings in FIRES patients and inconstant response to immunotherapy 12.
Likewise, FIRES is associated with monogenic mutations in PCDH19, SNC1A and POLG1, which are in turn associated with epileptic encephalopathies of similar characteristics: temporary association to infection, rapid onset of refractoriness, absence of encephalitis, and cognitive impairment markers in previously healthy patients 13-15. The presence of prolonged SE can be related to mitochondrial disorders etiologies and affect the reserve of energy and, therefore, neuronal function 11,16.
Autoimmunity plays an important role in epileptic disorders unrelated to infection or neoplasms 10,17 and in refractory epileptic encephalopathies with inconsistent findings of antibodies against neural surface antigens (antibodies anti-NMDA, anti-VGKC and anti-GAD) 3,4,10,11,18,19. The presence of antibodies and an excessive inflammatory response could justify the use of immunotherapy as a treatment for FIRES 3,16.
VKKL-ab, anti-GAD and GluR3 autoanti-bodies and oligoclonal bands in CSF are observed in a third of the patients 4. However, the reason for their presence, as well as their consequences and correlation with the phenotypic variants of FIRES, is unknown.
Manifestations and clinical course
FIRES usually develops in children under 15 years of age, who have fever between 2 and 1 4 days before the onset of symptoms, with a male-female ratio of 4:3. The febrile syndrome is attributed, mostly, to infections of the upper respiratory tract (more than 50%) and, in a smaller proportion, to gastroenteritis 4,20. Once epileptic seizures appear, a SE is established and then an SRSE-like behavior is observed; this SE usually lasts from 1 to 1 2 weeks 4. The seizures type at the beginning of the disease are mainly focal-motor, with clonic manifestations or segmental myoclonus that can compromise the face and other body segments 21,22, sometimes reaching up to 100 daily crises 23 (Figure 3).
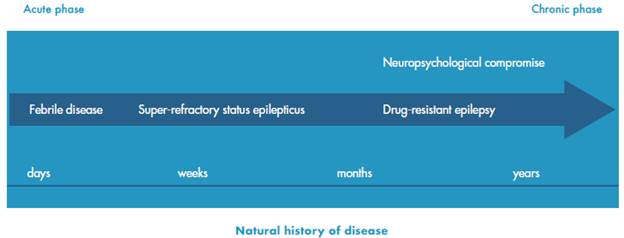
Source: Own elaboration based on Baalen et al24.
Figure 3 Natural history of the febrile infection-related epilepsy syndrome.
Except for the presence of burst suppression in barbiturate coma, EEG shows multi focal activity with greater involvement of the temporal and frontal lobes 4.
SRSE control in this patient may be related to the natural course of the disease and not to the pharmacological interventions.
With respect to neuroimaging, the variations observed in the MRI range between normal findings and different degrees of atrophy and hyperintensities in the temporal lobe, mainly in the hippocampal region 12,25,26, as well as in the insula and basal ganglia 11, perhaps secondary to prolonged neuronal activation 4.
Treatment of FIRES
Most of the medications indicated for this emergency, and even other non-usual medications such as carbamazepine or topiramate, have not shown a good response in the acute phase during status epilepticus 4,22,27,28. During this phase, barbiturates can be useful at high doses, achieving up to 50% of crisis control transiently; however, there are SE recurrences 27,29.
Other series 19,30 show that levetiracetam at regular doses for status epilepticus management could have a good response to control seizures in about half of FIRES patients. However, data are contradicted in another series of 12 patients 27, where control was not achieved with this medication. This treatment is effective in the long term to reduce seizures in more than 75% of patients at doses between 750 mg/d and 1500 mg/d, although adverse effects such as aggressiveness or impulsivity are frequent 31.
Immunomodulation
Plasmapheresis tests have been performed without obtaining a response; adrenocorticotropic hormone (ACTH), intravenous immunoglobulin (IVIG), and other steroids have shown responses in less than 5% of patients. There is no standardized scheme for the use of immunoglobulin in status epilepticus, so different treatment schemes have been used in these patients. In some cases 1.2 g IVIG were administered every 10 and 14 days, in others it was administered every 21 days for 4, 6 and 8 months or was used at a dose of 30 mg/Kg/day over 5 days, followed by 1 mg/kg/day; however, the dose used in many of the reports is not clear 4,22,26,27,28.
Ketogenic diet
Different series of FIRES patients have described the use of KD, finding a good response in terms of crisis control, thus turning this option into a promising therapy. A smaller proportion of patients did not respond and others presented recurrence when suspending it 21,26,27,32-34.
Mikaeloff et al.21 used KD in two FIRES patients; one of them had more than 100 seizures per day and achieved cessation of epileptic activity two days after initiating the treatment 21.
The use of KD in a study of 9 patients showed that 8 of them achieved ketonuria 2 to 4 days after initiating the diet, while seizures stopped in 7 of them 2 to 4 days after achieving ketonuria. Patients who responded to the treatment regained consciousness 24-58 hours after the cessation of seizures and motor functions in the following weeks. In one patient, KD was interrupted abruptly, which made the status recurrent in a short period of time and caused death 10 days later 33.
Vaccarezza et al.34 reported the use of KD in three patients with possible FIRES who presented SE of 52, 30 and 18 days of evolution, in whom SE stopped after 24 hours in the first two cases, and after 3 days in the last. After establishing KD, the first patient remained free of crisis, the second had ten partial seizures per month and the third had a crisis per month, evidencing a good response to this therapeutic measure.
During the chronic phase, one study assessed six patients treated with KD for a period between 6 months and 2 years, with a marked decrease in the frequency of seizures and recurring once or twice a week 33.
Other therapies
During the acute phase, no response has been obtained with medications such as lacos-amide, lidocaine, ketamine, verapamil, magnesium, vitamin B6, folic acid, biotin, paraldehyde or dextromethorphan 4,35. However, hypothermia has reported an acceptable response and fewer sequelae 36. Electroconvulsive therapy (ECT), as described by Mirás-Veiga et al.37 in one patient, also showed a decreased epileptiform activity.
IVIG has not shown good results during the chronic phase of FIRES, so other treatments such as surgical management have been included, with 50% reduction of seizures 27.
In the Howell series, six patients who overcame the acute phase of FIRES presented with drug-resistant epilepsy. The vagus nerve stimulator was used in two patients, obtaining a reduction of 30-40% of seizures; one was treated with immunotherapy, without obtaining a good response 18.
PROGNOSIS
A third of patients die, usually, 4-8 months after the acute phase or suffer from refractory epilepsy and intellectual disability 3,4,21,31; only a few survive the episode without any neurological sequelae 21,31,32,38,39. Of 68 patients followed in the series published by Kramer et al.4, 12 (18%) developed attention deficit or learning problems, 11 (16%) borderline cognition, 26 (38%) mild-moderate intellectual disability and 11 (16%) were in a vegetative state; some presented secondary peripheral neuropathy and ataxia.
Only KD, even used tardily, has shown efficacy in at least 50% of patients. The crises decrease during the following weeks or months, but patients remain with a cognitive deficit.
Regarding cognitive evolution, a follow-up report of two patients with FIRES treated with multiple medications showed emotional lability and significant alteration of memory, including working memory, evocation of words and auditory verbal narrations, as well as tangentiality during conversations and phonetic and verbal fluency alterations 12.
The use of barbiturates during the acute phase is significantly related to cognitive sequelae in memory, expressive language or executive function, as well as motor disability 3,31,36. About 15% of patients have a good evolution in the medium term even though FIRES is considered a "catastrophic syndrome" 4.
Early suspicion of this etiology, considering the absence of efficient diagnostic tests, may favor the timely establishment of KD, which shows the best results. Perhaps, the rapid control of crisis and the lower frequency of complications in the acute phase favor a better prognosis for the patient in the long term.